Management of Vesicoureteral Reflux
Christopher S. Cooper, M.D.
Kathleen Kieran, MD
Douglas W. Storm, MD
Pediatric Urology, The University of Iowa
Iowa City, Iowa
→ Enlace a la versión en español
Christopher S. Cooper, M.D.
Kathleen Kieran, MD
Douglas W. Storm, MD
Pediatric Urology, The University of Iowa
Iowa City, Iowa
→ Enlace a la versión en español
INTRODUCTION
Vesicoureteral reflux (VUR) is the retrograde flow of urine from the bladder into the ureters and possibly up to the kidneys. Reflux has been linked to an increased risk of pyelonephritis, renal insufficiency and hypertension, and is cited as the most common cause of end-stage renal disease in children [1-3]. Management of reflux is aimed at reducing the development of these adverse effects through maintenance of sterile urine, treating any associated bowel and bladder dysfunction, and in some cases operative correction of reflux.
Those managing VUR need to be familiar with several recent controversial issues. Although it appears that reflux management has improved outcomes for some children such that the incidence of reflux-associated nephropathy continues to decline, recent urologic literature suggests that the natural history of VUR does not follow a uniform pathway, and many children with reflux do not benefit from either diagnosis or treatment [4]. The condition in these children is self-limited and causes no adverse effects; however, a subset of children will benefit from both diagnosis and treatment. Clarification of which children constitute this subset remains the greatest challenge to the advancement of vesicoureteral reflux management.
BACKGROUND, INCIDENCE AND ETIOLOGY
VUR occurs in approximately 1-3% of children and is associated with 7-17% of children with end-stage renal disease worldwide [5-8]. The goal of VUR treatment is to prevent pyelonephritis and its sequelae such as renal parenchymal injury, hypertension, and chronic renal insufficiency. Permanent renal injury resulting from pyelonephritis, manifested as renal scarring on 99mTc dimercaptosuccinic acid (DMSA) scanning, may be detectable radiographically during or shortly after an acute episode of pyelonephritis, but in some cases, as long as 30-40 years have been reported between the first renal-scarring pyelonephritis and the development of hypertension or end-stage renal disease [9]. It is not clear if, in some cases, the poor renal outcome reflects intrinsic congenital dysplastic parenchymal abnormalities associated with but not caused by VUR, or if delayed recognition of urinary tract infections (UTI) and/or vesicoureteral reflux played the primary role [1]. Regardless, the prolonged time between the apparent initial renal insult and the clinically apparent sequelae underscores the need for long-term follow-up of patients if we are to identify children who will benefit from diagnosis and treatment of VUR.
Urinary tract infections are common in children, affecting about 5% of girls and about half as many boys [10]. An estimated 30-40% of children under the age of 5 who develop urinary tract infections have VUR upon further evaluation [8,11]. VUR can be categorized as either primary or secondary. Primary VUR is frequently attributed to an abnormally short intravesical tunnel at the ureterovesical junction, with shorter intravesical tunnels being associated with more severe reflux [12]. Secondary VUR develops when abnormal lower urinary tract function and elevated intravesical pressures lead to retrograde flow of urine¸ and is associated with conditions such as posterior urethral valves, urethral obstruction, or neurogenic bladder dysfunction. Secondary VUR may also be seen in children with no anatomic genitourinary or neurologic abnormality, but suffer from bladder and bowel dysfunction [13]. The patient must be carefully evaluated for underlying secondary causes of reflux, which, left untreated, may hamper spontaneous VUR resolution, predispose patients to urinary tract infections, and increase the risk of renal damage. This evaluation is important as the most frequent cause of secondary reflux is likely bowel and bladder dysfunction.
The International Reflux Study classified VUR along a 5-point scale defined by the degree of retrograde urine flow and the accompanying distortion of the pyelocalyceal system (Figure 1) [14]:
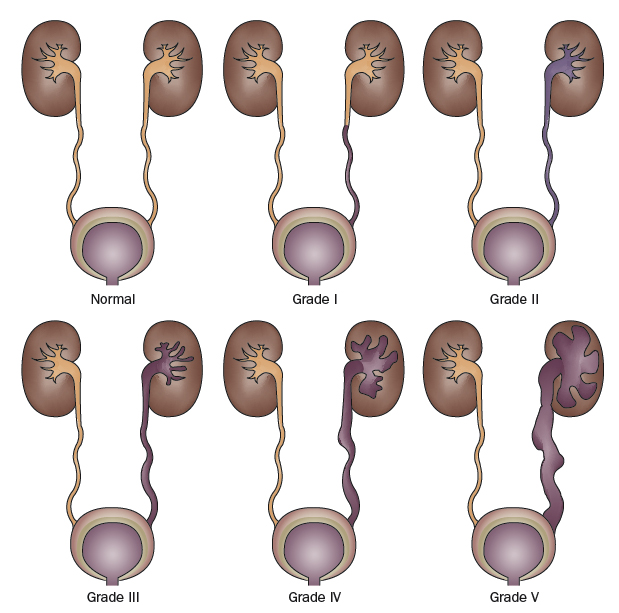
The severity or grade of VUR has been recognized as the main factor determining the likelihood of spontaneous reflux resolution and risk of renal injury. Higher grades of reflux are associated with decreased spontaneous resolution rates and increased prevalence of renal scars [10,15].Recently, additional factors influencing reflux resolution have been identified, which enable clinicians to refine the estimated likelihood of spontaneous reflux resolution in patients with radiographically similar VUR. Variables shown to be predictive of reflux resolution and/or the risk of renal injury include age, gender, laterality, bladder volume at the onset of reflux, bladder pressure at the onset of reflux, whether reflux occurs in the filling or voiding phase, presence of renal scars, presence of voiding dysfunction, and a history of urinary tract infections [16-23].
VUR as a factor predisposing to renal injury due to infection was first recognized in spinal cord injury patients. After surgical correction, children had fewer problems with pyelonephritis and urosepsis [24]. This association led to the discovery of VUR as a primary factor in the development of pyelonephritis. Further studies demonstrated a lower rate of new renal scars developing in children with primary reflux compared to those with secondary reflux due to neurogenic bladders or voiding dysfunction [25,26].
An understanding of the function of the lower urinary tract in children with VUR is of utmost importance. Children with bowel or bladder dysfunction not only have a higher incidence of breakthrough infections while on prophylaxis, they have more renal scarring, a lower spontaneous resolution rate, and a higher failure rate following surgery than children with “normal” elimination habits [1]. Thus, apparent primary VUR in children with neurogenic or non-neurogenic bladder and bowel dysfunction may behave similarly to those children with secondary VUR. Since the probability of reflux resolution or renal injury with secondary reflux is dependent on the bladder abnormality, it is now recognized that secondary VUR is more appropriately managed by addressing the lower urinary tract dysfunction. Details on the management of the neurogenic or non-neurogenic bladder dysfunction is beyond the scope of this chapter which focuses on primary reflux, but practitioners should carefully evaluate children with VUR for bladder/bowel dysfunction in order to optimize resolution rates, minimize breakthrough infections, and improve therapeutic outcomes.
INHERITANCE AND ROLE OF SCREENING
A strong inheritance pattern exists with primary VUR, with up to 80% of identical and 35% of fraternal twins concordant for presence of VUR [2]. While the exact gene has not been identified, the mode of transmission is thought to be autosomal dominant with variable penetrance. Multiple polymorphisms associated with abnormalities in ureteral budding have been identified in patients with primary VUR, although no single gene has been found to predominate [27]. The probability of a non-twin sibling having reflux is about 25% and the chances of offspring having reflux are approximately 35-50% [28,29]. Interestingly, this relationship is not as strong in children with dysfunctional elimination [30]. The utility of sibling screening for reflux is questionable [30,31]; however, one study has noted that renal damage is more prevalent in screened siblings with VUR and a history of UTI compared to those without a history of UTI [32]. This finding is consistent with the known increased risk of renal scars developing in children with febrile UTIs who have reflux. It remains to be demonstrated whether detection and management of VUR in an asymptomatic screened sibling will result in significantly decreased adverse sequelae; therefore, no consensus regarding the practice of asymptomatic sibling screening for VUR currently exists. Until recently, many practitioners routinely screened siblings with a voiding cystourethrogram; however, the AUA recently issued an update to its Clinical Guidelines for the Management of Primary Vesicoureteral Reflux in Children [33], with a current recommendation that siblings be screened with a renal-bladder ultrasound (RBUS), with VCUG reserved for those in whom the RBUS is abnormal.
A lack of consensus also exists regarding the need to screen newborns with a history of low-grade prenatal hydronephrosis for VUR. One study demonstrated that in children with a history of prenatal hydronephrosis who had persistent Society for Fetal Urology grade II hydronephrosis after birth, identification of VUR and use of prophylactic antibiotics significantly reduced the risk of febrile UTI [34]. Others suggest that routine screening and treatment of asymptomatic siblings of those with prenatal hydronephrosis provides little, if any, benefit and is not cost-effective [35,36]. These controversies emphasize that appropriate clinical assessment and management of VUR requires an individualized approach that considers multiple factors.
Timing of evaluation for possible VUR in patients with a history of febrile UTI remains a hotly debated topic. In 2011, the American Academy of Pediatrics issued a Clinical Practice Guideline based on a meta-analysis of multiple studies of the evaluation, treatment, and outcome of VUR [37]. They concluded that a VCUG may be deferred until after the second febrile UTI in children aged 2-24 months with a normal renal ultrasound. This recommendation was based on data suggesting that the rates of urinary tract infections were unchanged in patients with VUR prescribed antibiotic prophylaxis compared to those not prescribed antibiotics. Further review of this data suggests that the subset of these patients with grades 3, 4, and 5 VUR do appear to benefit from antibiotic prophylaxis and therefore some patients with a febrile UTI would benefit from a VCUG after their first febrile UTI. As discussed further below, the recommendations of the American Academy of Pediatrics have been strongly contested by the American Urological Association and the pediatric urology community, and at present the authors continue to recommend follow-up radiographic evaluation of children presenting with even a single febrile urinary tract infection.
CLINICAL MANAGEMENT
There is no universal optimal management for children with VUR. As previously noted, multiple anatomic and physiologic variables influence the likelihood of spontaneous VUR resolution, while extrinsic factors such as patient and family preferences, medication compliance, social situations and urinary tract infection rates must also be considered when developing a management plan for a particular child. Keeping in mind that the management of VUR should be individualized to each child after consideration of the multiple intrinsic and extrinsic factors which influence outcome, we discuss the various treatment options below.
Antibiotic Prophylaxis versus Operative Intervention
The daily administration of low-dose antibiotics in children with VUR is based on the knowledge that spontaneously resolution rates for primary VUR are very high (even for severe VUR in selected populations), and that new reflux-associated renal scarring appears to occur exclusively in the setting of infected urine, particularly in the poles of the kidneys where the intrarenal collecting system is more likely to have compound calcyes [38]. Thus, maintenance of sterile urine until spontaneous reflux resolution may avoid the morbidity of surgery and renal scarring. Operative intervention is generally reserved for children with breakthrough UTI while on antibiotic prophylaxis, worsening renal function, or those in whom other considerations favor definitive intervention over daily antibiotic administration. Several studies suggest that children on antibiotic prophylaxis without breakthrough infections or evidence of renal injury can be safely observed without antibiotic prophylaxis or correction of VUR, once they have reached an age and developed bowel and bladder habits when urinary tract infections are less likely [39,40].
Several large prospective studies have attempted to address the efficacy of operative intervention versus antibiotic prophylaxis in children with reflux. These studies have generally shown no significant difference in renal function or growth, the progression or development of new scars, or UTIs [10,41-44]. However, pyelonephritic symptoms, including febrile UTIs, tended to be more common in the medically treated groups [45-47]. In general, children who ultimately underwent surgical intervention tended to develop renal scars at an earlier age than those treated medically, but no significant difference occurred overall with longer follow-up in terms of new renal scars in those treated with antibiotics compared to operatively [8,48]. These observations suggested that a potential benefit might be a reduction in pyelonephritis from the antireflux operations for some patients; however, other researchers suggested that, once renal scarring occurs, the disease tends to run its course and operative treatment has little benefit [9,49]. One review concluded that nine reimplantations would be required to prevent one febrile UTI, with no reduction in the number of children developing any renal damage [8], again reinforcing the need to better define which children with VUR may benefit from intervention.
Antibiotic Prophylaxis versus Observation
Many question the need for antibiotic prophylaxis, suggesting that, in select individuals, the chance for pyelonephritis and renal damage off prophylactic antibiotics is small [39,40,45,50]. Between 30% and 50% of children with a UTI will suffer from recurrent infections and because the diagnosis of reflux often follows a urinary tract infection, it leads many individuals to the erroneous assumption that the reflux is responsible for the infection. However, it does not significantly predispose to urinary tract infections unless it is a high enough grade to induce significant urinary stasis [40,42]. More often, UTIs are due to predisposing conditions such as a previous history of urinary tract infections, female gender, constipation, infrequent voiding, incomplete bladder emptying, and impaired immunity.
In general, daily antibiotic prophylaxis appears to be safe and well tolerated, but it does incur cost and potential risks. Antibiotic prophylaxis for urinary tract infections has been associated with a 24-fold increased risk of trimethoprim-sulfamethoxazole resistant Escherichia coli [51]. Other studies have demonstrated the emergence of other bacteria with high rates of resistance in children receiving prophylactic antibiotics [52]. While some contend that antibiotic prophylaxis is effective and safe for preventing urinary tract infections [53], antibiotic prophylaxis has not been proven to reduce pyelonephritis in all children with VUR. Interestingly, a 2010 study suggested that the compliance rate for merely filling the prescription was only 40%, suggesting that many patients placed on antibiotic prophylaxis never receive the medication [54].
A number of randomized studies have attempted to evaluate the efficacy and adverse effects of antibiotic prophylaxis in children with VUR [6,53,55,56]. These studies have generally failed to show a significant reduction in acute pyelonephritis or renal scars in children with VUR being treated with antibiotic prophylaxis. Some studies actually reported an increase in UTIs in children on antibiotics as well as an increase in antibiotic resistant bacteria causing the UTIs [6,53,55]. By subset analysis, other studies identified younger age and increasing grade of reflux as risk factors for recurrent febrile UTIs [53,57]. The current RIVUR trial, a National Institutes of Health-funded multicenter, randomized, placebo-controlled trial is evaluating whether prophylactic trimethoprim-sulfamethoxazole is superior to placebo for children aged 2 to 72 months diagnosed with grades I through IV VUR after a first or second UTI [1]. This large trial of 600 children is expected to confirm the findings of the smaller studies previously noted.. It is also hoped that subset analysis will identify characteristics of children at greatest risk for recurrent pyelonephritis and development of new scars. Unfortunately, as previously noted, the lag time between development of renal injury and apparent clinical sequelae of hypertension or renal insufficiency will require decades of follow-up to characterize the group of children who may truly benefit from intervention.
CLINICAL EVALUATION
Assessment of Reflux: Voiding Cystourethrography and Nuclear Cystogram
The only tests that routinely detect reflux reliably are voiding cystourethrography (VCUG) and nuclear cystography. An initial VCUG is an appropriate initial test because it provides better anatomic details, including the presence or absence of periureteral diverticula, ureteral duplication, and abnormalities of the lower urinary tract such as trabeculations or urethral obstruction. It also allows for more precise grading of reflux than a nuclear cystogram. If a VCUG demonstrates no significant anatomic abnormalities, nuclear cystography may be performed in order to limit radiation exposure. Although the nuclear cystogram is frequently considered a more sensitive test, the ordering practitioner should be aware that each type of cystogram has limitations to its ability to detect reflux in a given population [58]. The amount of radiation exposure during a given test is dependent on multiple factors, including patient size, distance from the radiation source, and duration of fluoroscopy. The American Radiological Association promotes the ALARA (as low as reasonably achievable) concept in guiding the number and nature of radiographic studies obtained in children [59], and efforts are routinely made to limit the number of snapshots and the total fluoroscopy time during these tests. Despite concern regarding exposure of the pelvic organs to ionizing radiation with childhood cystograms, there are presently no data suggesting deleterious effects on future effect on gonadal function or an increased risk of pelvic malignancies.
Nuclear cystography reliably detects all grades of vesicoureteral reflux and may be more sensitive for the detection of intermittent VUR [60].48 At the author’s institution bladder pressures are routinely monitored through a dual lumen catheter during the filling phase of a nuclear cystogram providing a nuclear cystometrogram [22]. This technique permits the measurement of the intravesical pressure at the onset of reflux as well as other changes suggestive of a non-compliant bladder or bladder dysfunction (Figure 5A,B).
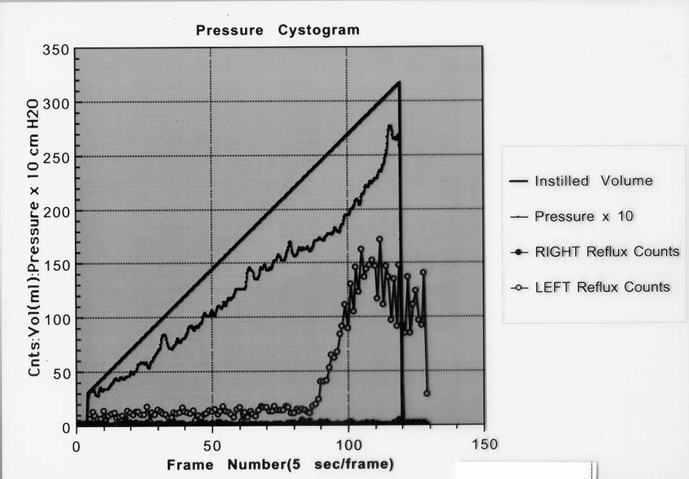
Fig 5A
Fig 5B
Bladder pressure at the onset of reflux has been demonstrated as a predictor of spontaneous VUR resolution independent of grade [21]. In addition to higher bladder pressure, independent predictors of VUR resolution on nuclear cystometrogram included the onset of reflux occurring at greater instilled bladder volumes [20].
Catheterization can be a traumatic experience for a young child. Efforts to decrease the traumatic nature of the procedure include the use of lubricants containing local anesthetics, use of child life specialists, and conscious sedation. Decision-analysis tools [61] and other radiographic modalities such as ultrasound [62] and magnetic resonance imaging [63] have been employed with some success in detection of VUR, but their sensitivity lags behind cystograms, and so the rate of adoption has been low. Typically, cystograms are repeated annually; however, it has been suggested that in children less likely to resolve VUR, the interval between cystograms should be lengthened to decrease exposure to radiation, the number of traumatic studies, and cost [64]. Recent advances in the ability to predict timing of spontaneous resolution should aid in determining follow-up time to minimize the number of repeat cystograms [19,20,65].
Assessment of Renal Scars
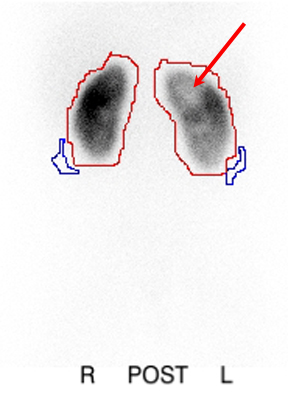
Renal scarring is believed to occur when infected urine comes in contact with renal parenchyma; it has been associated with an increased risk of hypertension, proteinuria, and renal insufficiency [3,66]. In a meta-analysis examining the presence of renal damage in children hospitalized with urinary tract infection, one study found that approximately 34% of children with pyelonephritis had VUR, and of those with VUR and pyelonephritis, 72% had an abnormal DMSA scan [67]. DMSA scintigraphy, in which the radioactive agent binds to the proximal tubules, has been found to be a more sensitive study than intravenous pyelogram (IVP) for the detection of reflux nephropathy [68]. The scan can also detect changes of acute pyelonephritis with greater sensitivity and specificity than CT scan, magnetic resonance imaging, or ultrasound [69] (Figure 6).
Mercaptoacetyltriglycine (MAG3) has an improved ability to detect renal scarring compared to IVP. Renal scans permit determination of relative renal function and multiple reports demonstrate a high degree of correlation with respect to relative renal function determined with different types of renal scans [70]. The DMSA scan is considered by many to be the most sensitive test for renal scar detection; however, the reported sensitivity rate of the MAG3 compared to the DMSA varies from 88% to equal to or slightly better than a DMSA scan [70-72]. Advantages of MAG3 include lower radiation exposure, cost, and time requirement, as well as visualization of the collecting system which may improve specificity compared to DMSA in those with significant dilation of the collecting system [70].
Reflux Nephropathy/Renal Scarring
At least one-third of VUR patients have renal scars [46,73] and the risk of scarring may be highest with the first febrile UTI. The presence of scarring implies regions of renal damage and increases the risk for long-term adverse sequelae. In newborns with VUR, scars have been detected in association with high-grade reflux before the occurrence of clinical UTI [74]. These “congenital scars” are thought to be regions of focal dysplasia or hypoplasia resulting from abnormal nephrogenesis as opposed to damaged normal tissue following pyelonephritis. Since DMSA scans are not routinely performed on neonates, it is unknown what proportion of renal scars attributed to infectious injury are actually due to abnormalities of embryogenesis [75]. The ultimate significance of these regions of dysplasia remains to be determined; however, they are regions of diminished renal function and can be associated with significant morbidity and mortality independent of the development of infection-associated renal scarring [76].
Children with renal scars are more likely to develop additional scars than children without renal scars [77-79]. One retrospective study of 120 patients demonstrated a significantly higher chance of developing a breakthrough urinary tract infection in those with grades III-V reflux and an abnormality on baseline DMSA scan (60%) compared to those without an abnormality (6%) [80]. In one study with a mean follow-up of 12 years after an anti-reflux operation, children with unilateral renal scars had an 11% chance of developing hypertension and an 18.5% chance if they had bilateral renal scars [81]. Others have suggested the incidence of hypertension in children with bilateral renal scars is about 20% [82]. Children with severe bilateral renal scars are significantly more likely to develop proteinuria, chronic renal insufficiency and renal failure than those with unilateral scars or unscarred kidneys [83,84]. These data strongly suggest that children with renal scars are at increased risk for further development of scars and long-term clinical sequelae.
VUR Grade and Renal Scarring
Children with VUR and UTIs are at an increased risk for renal scarring compared to children with UTI and no reflux. In the International Reflux Study, 50% of children with grades III or IV reflux had scars at study entry [68]. Multiple studies demonstrate a direct correlation between increased prevalence of renal scarring and higher grades of reflux [85]. Renal scarring develops less often in non-dilating forms of reflux [76,77,86]. The chance of developing further renal parenchymal loss has also been shown to be higher in children with grade III-V reflux than in those with grade I-II [44,87]. Renal scars have also been demonstrated to be a negative predictor of VUR resolution independent of reflux grade [17].
The association of renal scars with higher grades of reflux and risk for subsequent scars as well as decreased resolution rates lead some to conclude that the standard initial evaluation of a child with a febrile UTI should begin with a renal scan and not a VCUG (the “top-down” approach). Only in those children with an abnormal scan should a VCUG be obtained. The benefit of such an approach would be a reduction in the number of children undergoing VCUG and the identification of reflux in a higher risk group; in theory, patients with reflux but no structural or functional renal abnormalities might go undiagnosed with this approach, but the absence of such renal abnormalities suggests that the reflux is not likely to be clinically significant. The use of an ultrasound instead of a renal scan has not been accepted because of the decreased sensitivity of the ultrasound. One study noted that up to 25% of patients with cortical defects on DMSA had a normal ultrasound, providing further data on the utility of renal scans in the evaluation of children with febrile UTIs [88].
OPERATIVE MANAGEMENT
Endoscopic Treatment
Following FDA approval of the use of dextranomer/hyaluronic acid copolymer (Dx/HA) (Deflux®, Q-Med, Uppsala, Sweden) for the treatment of primary VUR, parents and physicians began to view endoscopic treatment as an alternative to starting their child on an undefined duration of prophylactic antibiotics [89]. In the first 5 years after its approval, the number of patients undergoing endoscopic antireflux surgery doubled, with no corresponding change in the number of patients undergoing open ureteral reimplantation [90]. Dx/HA is the only FDA approved commercially available injectable treatment for reflux in the United States. It is a synthetic mixture of dextran microspheres in a hyaluronic acid gel. The dextranomer particle size is larger than that of polytetrafluoroethylene particles which should prevent the complication of lymphatic migration previously demonstrated with polytetrafluoroethylene [91].
Although the reflux resolution rate is not as high as that with open ureteral reimplantation, endoscopic correction of VUR offers a minimally invasive, outpatient procedure with a low risk of complications. While it is a seemingly simple procedure, several studies have demonstrated a learning curve with improved results obtained with increasing experience [92-94]. Other factors associated with successful endoscopic correction include lower reflux grade, lack of lower urinary tract voiding dysfunction symptoms, increased volume of Dx/HA injected, visual assessment of mound configuration following injection, and surgical technique [94-96].
In the short term, VUR resolution rates for a single ureter treated with Dx/HA range from 59% to 95% [94]. If patients undergo a second injection for persistent VUR, the success rate is improved, but a third injection is rarely curative [89,92,97]. The long-term durability of successful endoscopic correction of reflux is not well documented. There is a single long-term study documenting a persistent 95% efficacy for polytetrafluoroethylene with a mean follow-up of 14 years [98]. Most studies of Dx/HA only report the results of cystograms at three months or one year following the procedure. In studies using bovine-cross-linked collagen, the high failure rate did not occur until years later, raising concerns regarding the long-term efficacy of Dx/HA [99]. Despite the relatively short-term follow-up, recurrent reflux rates between11% and 26% between 3 and 12 months following a single Dx/HA acid copolymer injection have been reported [100,101]. One longer-term study of 49 patients demonstrated resolution of reflux at both three and 12 months after injection, but 13% recurred at a mean of three years post-injection [92]. It is unclear if reflux recurrence following initial successful injection of Dx/HA is related to technical (e.g. injection technique and learning curve), patient (e.g. elimination habits), or Dx/HA characteristics (e.g. mound migration and shrinkage), or some combination of these.
The uncertainty over long-term success with Dx/HA may suggest a need for long-term monitoring of children undergoing this treatment. Some have suggested that monitoring should only be considered for those who have recurrent febrile UTIs. Aside from potential failure, calcification of the implant secondary to a foreign body reaction has recently been reported as another potential long-term factor [102]. Mound calcification is not unique to Dx/HA and has been described with many endoscopically injected agents [103,104]. However, calcification can be confused with ureterolithiasis, and providers must maintain a high level of suspicion for this entity to avoid unnecessary diagnostic tests and surgical interventions [105]. The likelihood of ureteral obstruction following Dx/HA appears to be about 0.6% [106], and typically requires ureteral reimplantation with excision of the affected segment.
Ureteral Reimplantation
The surgical treatment of vesicoureteral reflux has evolved over the last five decades. A lower abdominal transverse incision is typically used, leaving a small scar in the skin crease that frequently becomes inconspicuous. Multiple different techniques for ureteral reimplantation have been performed, with the primary difference being intravesical surgery, where the bladder is opened and the ureters are dissected intravesically, versus extravesical reimplantation, where the ureters are dissected away from the bladder wall without opening the bladder, are left attached to the bladder mucosa, and reimplanted under flaps of bladder muscle
(Figure 7).
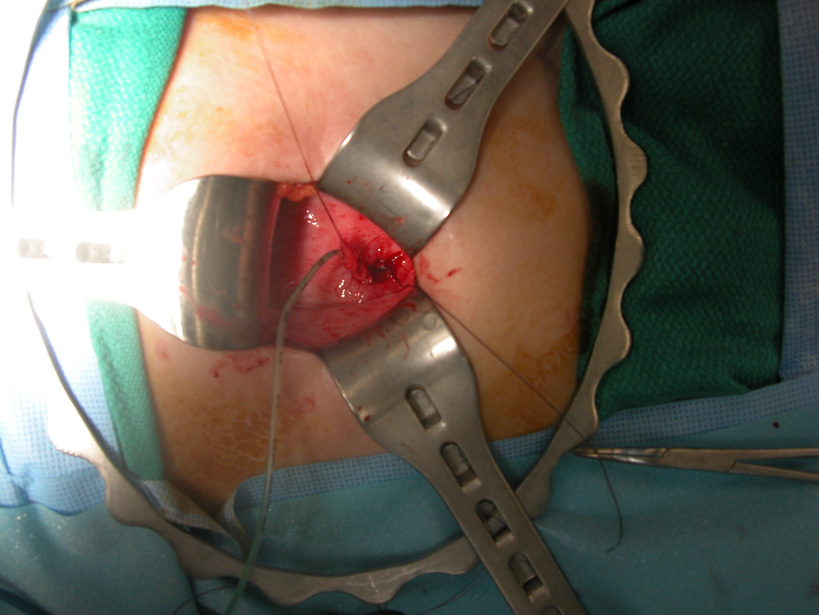
There is no clear documentation of any of particular technique being superior [10], and selection of a given technique is typically individualized to the child at the discretion of the operating physician.
Advancements in analgesia, surgical techniques, and the understanding that children undergoing ureteral reimplantation for primary VUR rarely need ureteral stents or prolonged bladder drainage has improved the length of stay and decreased the morbidity of the procedure [107]. Several series report patients undergoing both intravesical and extravesical ureteral reimplantation surgery as outpatients [108-110]. Recent studies have documented the use of laparoscopic and robotic ureteroneocystotomy in attempts to further reduce perioperative morbidity [111]. Results of multiple series document success rates with open ureteral reimplantation of greater than 95% and close to 100% for lower grades of reflux [112-114]. The procedures do carry risks of anesthesia and potential complications including ureteral obstruction, persistent reflux, infection and bleeding [10]. General tenets of ureteral reimplantation include minimizing ureteral handling, excision of the intravesical ureteral segment, development of a tunnel at least five times the diameter of the ureteral lumen, and creation of a tension-free anastomosis. In large ureters, tailoring through either excision or plication may be necessary to facilitate achievement of an adequate intravesical tunnel.
INDIVIDUALIZED MANAGEMENT
While VUR itself can be simply defined, more detailed research confirms that VUR is not a single condition but rather occurs with wide variations in severity and impact. The answers to many questions regarding reflux remain unknown; however, it is clear that definitive treatment and even diagnosis of VUR is of questionable clinical benefit for many patients. Currently, the decision to perform surgery or to continue or stop antibiotic prophylaxis is based on physician and parent assessment of risks and benefits as we currently understand them. Although the decision to pursue surgery has traditionally been based predominantly on the grade of reflux, a truly informed decision must consider multiple other variables, such as patient age, gender, history of UTIs, renal status, and likeliness and timing of spontaneous resolution. A patient’s social situation and parental preferences, as well as willingness to comply with either conservative management or postoperative care, must also be taken into consideration.
Multiple prognostic factors relative to a child’s chance for spontaneous reflux resolution have been defined [16,17,20,21]. One of the predictive factors independent of VUR grade identified in these studies included the instilled bladder volume at the onset of reflux. Reporting the bladder volume at the onset of reflux as additional predictive information should become standard practice for those performing VCUGs or nuclear cystograms.
Attempting to determine the likelihood and timing of spontaneous VUR resolution in a particular child while taking into account multiple prognostic variables such as age, gender, grade of VUR, bladder volume at onset of reflux, presence of dysfunctional voiding, history of UTIs, laterality and duplication is extremely complex. To this end, a user-friendly neural network that incorporates all these predictive factors is available for use at http://godot.urol.uic.edu/urocomp/svm_vur.html
(Figure 8) [19].
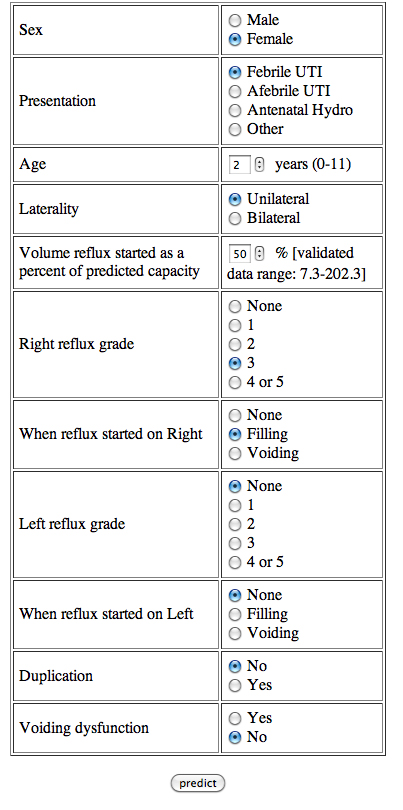
The use and accuracy of this model was validated on an international basis in a group of Japanese children [65]. For children who have had a renal scan, a second computer model was generated incorporating the additional renal scan data to improve prognostic accuracy and is available at the same website [20].
FUTURE DIRECTION
Although improved accuracy regarding the likelihood and timing of spontaneous VUR resolution permits better management decisions, additional data is needed. Further studies are needed to define an individual child’s risk of subsequent pyelonephritis, renal damage, and ultimately clinical sequelae. Prospective studies such as the RIVUR trial should help define which children benefit from antibiotic prophylaxis, but retrospective studies assessing long-term outcomes in adults with a history of VUR should be pursued to better define those at greatest risk for development of clinical sequelae. Though somewhat limited relative to prospective studies, well designed retrospective studies may minimize these limitations and have the advantage of providing needed information now as opposed to decades from now.
References
1. Mathews R et al. Controversies in the management of vesicoureteral reflux: the rationale for the RIVUR study. J Pediatr Urol 2009;5:336-41.
2. Kaefer M et al. Sibling vesicoureteral reflux in multiple gestation births. Pediatrics 2000;105:800-4.
3. Smellie JM et al. Retrospective study of children with renal scarring associated with reflux and urinary infection. Br Med J 1994:308:1193-6.
4. Cooper CS et al. Vesicoureteral reflux: who benefits from surgery? Urol Clin North Am 2004;31:535-41.
5. Cooper CS et al. The outcome of stopping prophylactic antibiotics in older children with vesicoureteral reflux. J Urol 2000;163:269-73.
6. Garin EH et al. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics 2006;117:626-32.
7. Craig JC et al. Does treatment of vesicoureteric reflux in childhood prevent end-stage renal disease attributable to reflux nephropathy? Pediatrics 2000;105:1236-41.
8. Hodson EM et al. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev 2007;3:CD001532.
9. Winberg J. Management of primary vesico-ureteric reflux in children—operation ineffective in preventing progressive renal damage. Infection 1994;22(suppl 1):S4-S7.
10. Elder JS et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol 1991;157:1846-51.
11. Baker R et al. Relation of age, sex, and infection to reflux: data indicating high spontaneous cure rate in pediatric patients. J Urol 1966;95:27-32.
12. Paquin AJ. Ureterovesical anastomosis. The description and evaluation of a technique. J Urol 1959;82:573-83.
13. Koff SA. Relationship between dysfunctional voiding and reflux. J Urol 1992;148:1703-5.
14. Lebowitz RL et al. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr Radiol 1985;15:105-9.
15. Schwab CW et al. Spontaneous resolution of vesicoureteral reflux: a 15-year perspective. J Urol 2002;168:2594-9.
16. McMillan Z et al. Bladder volume at onset of reflux on initial cystogram predicts spontaneous resolution. J Urol 2006;176:1838-41.
17. Nepple KG et al. Abnormal renal scans and decreased early resolution of low grade vesicoureteral reflux. J Urol 2008;180:1643-7.
18. Nepple KG et al. Adding renal scan data improves the accuracy of a computational model to predict vesicoureteral reflux resolution. J Urol 2008;180:1648-52.
19. Knudson MJ et al. Computational model for predicting the chance of early resolution in children with vesicoureteral reflux. J Urol 2007;178:1824-7.
20. Knudson MJ et al. Predictive factors of early spontaneous resolution in children with primary vesicoureteral reflux. J Urol 2007;178:1684-8.
21. Van Arendonk KJ et al. Nuclear cystometrogram-determined bladder pressure at onset of vesicoureteral reflux predicts spontaneous resolution. Urology 2007;69:767-70.
22. Cooper CS. Bladder pressure at the onset of vesicoureteral reflux determined by nuclear cystometrogram. J Urol 2003;170:1537-40.
23. Arsanjani A. Identification of filling versus voiding reflux as predictor of clinical outcome. Urology 2007;70:351-4.
24. Hutch JA. Vesico-ureteral reflux in the paraplegic: cause and correction. J Urol 1952;68:457-69.
25. Sukamoto E et al. Reappraisal of Tc-99m DMSA scintigraphy for follow up in children with vesicoureteral reflux. Ann Nucl Med 1999;13:401-6.
26. Naseer SR et al. New renal scars in children with urinary tract infections, vesicoureteral reflux and voiding dysfunction: a prospective evaluation. J Urol 1997;158:566-8.
27. van Eerde AM et al. Genes in the ureteric budding pathway: association study on vesico-ureteral reflux patients. PLoS ONE 2012:7):e31327.
28. Noe HN. The long-term results of prospective sibling reflux screening. J Urol. 1992;148:1739–1742
29. Wan J et al. Sibling reflux: a dual center retrospective study. J Urol 1996;156:677-9.
30. Noe HN. The relationship of sibling reflux to index patient dysfunctional voiding. J Urol 1988;140:119-20.
31. Routh JC et al. Costs and consequences of universal sibling screening for vesicoureteral reflux: Decision Anal Pediatr 2010;126:865-71.
32. Pirker ME et al. Renal scarring in familial vesicoureteral reflux: is prevention possible? J Urol 2006;176:1842-6.
33. Skoog SJ et al. Pediatric Vesicoureteral Guidelines Panel summary report: clinical practice guidelines for screening siblings of children with vesicoureteral reflux and neonates/infants with prenatal hydronephrosis. J Urol 2010;184:1145-51.
34. Estrada CR et al. Vesicoureteral reflux and urinary tract infection in children with a history of prenatal hydronephrosis- should voiding cystourethrography be performed in cases of postnatally persistent grade II hydronephrosis? J Urol 2009;181:801-7.
35. Khoury A. Screening for reflux-what price reassurance? J Urol 2009;181:445-6.
36. Hsieh MH et al. Cost-utility analysis of treatment algorithms for moderate grade vesicoureteral reflux using Markov models J Urol 2007;77:703-9.
37. Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011:128:595-610.
38. Coulthard MG et al. Renal scarring caused by vesicoureteric reflux and urinary infection: a study in pigs. Pediatr Nephrol 2002;17:481-4.
39. Cooper CS et al. The outcome of stopping prophylactic antibiotics in older children with vesicoureteral reflux. J Urol 2000;163:269-73.
40. Thompson RH et al. Cessation of prophylactic antibiotics for managing persistent vesicoureteral reflux. J Urol 2001;166:1465-9.
41. Smellie JM. Commentary: management of children with severe vesicoureteral reflux. J Urol 1992;148:1676-8.
42. Birmingham Reflux Study Group. A prospective trial of operative versus nonoperative treatment of severe vesico-ureteric reflux: 2 years’ observation in 96 children. Contrib Nephrol 1984;39:169-85.
43. Birmingham Reflux Study Group. Prospective trial of operative versus nonoperative treatment of severe vesicoureteric reflux in children: five years’ observation. Br Med J 1987;295:237-41.
44. Duckett JW et al. Surgical results: International Reflux Study in Children—United States branch. J Urol 1992;148:1674-5.
45. Jodal U et al. Infection pattern in children with vesicoureteral reflux randomly allocated to operation or long-term antibacterial prophylaxis. J Urol 1992;148:1650-2.
46. Weiss R et al. Results of a randomized clinical trial of medical versus surgical management of infants and children with grades III and IV primary vesicoureteral reflux (United States). J Urol 1992;148:1667-73.
47. Elo J et al. Character of urinary tract infections and pyelonephritic renal scarring after antireflux surgery. J Urol 1983;129:343-6.
48. Belman AB. Vesicoureteral reflux. Pediatr Clin North Am 1997;44:1171-90.
49. Ransley PG et al. The pathogenesis of reflux nephropathy. Contrib Nephrol 1979;16:90-7.
50. Bailey RR. Commentary: the management of grades I and II (nondilating) vesicoureteral reflux. J Urol 1992;148:1693-5.
51. Allen UD et al. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. Can Med Assoc J 1999;160:1436-40.
52. Cheng C et al. Antibiotic resistance patterns of community-acquired urinary tract infections in children with vesicoureteral reflux receiving prophylactic antibiotic therapy. Pediatrics 2008;122:1212-7.
53. Montini G et al. Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics 2008;122:1064-71.
54. Copp HL et al; Urologic Diseases in America Project. Compliance with antibiotic prophylaxis in children with vesicoureteral reflux: results from a national pharmacy claims database. J Urol 2010;183:1994-9.
55. Pennesi M et al. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics 2008;121:e1489-e1494.
56. Roussey-Kesler G et al. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux: results from a prospective randomized study. J Urol 2008;179:674-9.
57. Conway PH et al. Recurrent urinary tract infections in children: risk factors and association with prophylactic antimicrobials. JAMA 2007;298:179-86.
58. McLaren CJ et al. Direct comparison of radiology and nuclear medicine cystograms in young infants with vesico-ureteric reflux. BJU Int 2001;87:93-7.
59. Strauss KJ et al. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients--a white paper executive summary. Pediatr Radiol 2006;36(suppl 2):110-2.
60. Lebowitz RL. The detection and characterization of vesicoureteral reflux in the child. J Urol 1992;148:640-2.
61. Leroy S et al. Prediction of moderate and high grade vesicoureteral reflux after a first febrile urinary tract infection in children: construction and internal validation of a clinical decision rule. J Urol 2012;187:265-71.
62. Novljan G et al. Ultrasound detection of vesicoureteral reflux in children. J Urol 2010;184:319-24.
63. Lee SK et al. Magnetic resonance voiding cystography in the diagnosis of vesicoureteral reflux: comparative study with voiding cystourethrography. J Magn Reson Imag 2005;21:406-14.
64. Arant BS. Editorial: vesicoureteral reflux and evidence-based management. J Pediatr 2001;139:620-1.
65. Shiraishi K et al. Validation of a prognostic calculator for prediction of early vesicoureteral reflux resolution in children. J Urol 2009;182:687-90.
66. Ransley PG et al. Reflux nephropathy: effects of antimicrobial therapy on the evolution of the early pyelonephritic scar. Kidney Int 1981;20:733-42.
67. Gordon I et al. Primary vesicoureteral reflux as a predictor of renal damage in children hospitalized with urinary tract infection: a systematic review and meta-analysis. J Am Soc Nephrol 2003;14:739-44.
68. Ellison BS et al. Comparison of DMSA scintigraphy with intravenous urography for the detection of renal scarring and its correlation with vesicoureteral reflux. Br J Urol 1992;69:294-302.
69. Madj M et al. Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA, SPECT, spiral CT, MR imaging, and power Doppler US in an experimental pig model. Radiology 2001;218:101-8.
70. Smokvina A et al. The renal parenchyma evaluation: MAG3 vs. DMSA. Coll Antropol 2005;29:649-54.
71. Sfakianakis GN et al. Diuretic MAG3 scintigraphy (F0) in acute pyelonephritis: regional parenchymal dysfunction and comparison with DMSA. J Nucl Med 2000;41:1955-63.
72. Gordon I et al. Can technetium-99-m-mercaptoacetyltriglycine replace technitium-99m-dimercaptosuccinic acid in the exclusion of a focal renal defect? J Nucl Med 1992;33:2090-3.
73. Smellie JM. Reflections on 30 years of treating children with urinary tract infections. J Urol 1991;146:665-8.
74. Nguyen HT et al. 99m technetium dimercapto-succinic acid renal scintigraphy abnormalities in infants with sterile high grade vesicoureteral reflux. J Urol 2000;164:1674-8.
75. Patterson LT et al. Acquired versus congenital renal scarring after childhood urinary tract infection. J Pediatr 2000;136:2-4.
76. Bailey RR et al. Long-term followup of infants with gross vesicoureteral reflux. J Urol 1992;148:1709-11.
77. Ylinen E et al. Risk of renal scarring in vesicoureteral reflux detected either antenatally or during the neonatal period. Urology 2003;61:1238-43.
78. Lenaghan D et al. The natural history of reflux and long-term effects of reflux on the kidney. J Urol 1976;115:728-30.
79. Olbing I et al. Renal scars and parenchymal thinning in children with vesicoureteral reflux: a 5-year report of the International Reflux Study in Children (European branch). J Urol 1992;148:1653-6.
80. Mingin GC et al. Abnormal dimercapto-succinic acid scans predict an increased risk of breakthrough infection in children with vesicoureteral reflux. J Urol 2004;172:1075-7.
81. Wallace DM et al. The long-term follow-up of surgically treated vesicoureteric reflux. Br J Urol 1978;50:479-84.
82. Edwards D et al. Disappearance of vesicoureteric reflux during long-term prophylaxis of urinary tract infections in children. Br Med J 1977;2:285-8.
83. Jodal U et al. Guidelines for management of children with urinary tract infection and vesico-ureteric reflux. Recommendations from a Swedish state-of-the-art conference. Acta Paediatr Suppl 1999;88:87-9.
84. Mor Y et al. Analysis of the long-term outcome of surgically corrected vesico-ureteric reflux. BJU Int 2003;92:97-100.
85. Hoberman A et al. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 2003;348:195-202.
86. Rolleston GL et al. Relationship of infantile vesicoureteric reflux to renal damage. Br Med J 1970;1:460-3.
87. Berg UB. Long-term followup of renal morphology and function in children with recurrent pyelonephritis. J Urol 1992;148:1715-20.
88. Hamoui N et al. Ultrasound fails to delineate significant renal pathology in children with urinary tract infections: a case for dimercapto-succinic acid scintigraphy. J Urol 2008;180:1639-42.
89. Molitierno JA et al. Endoscopic treatment of vesicoureteral reflux using dextranomer hyaluronic acid copolymer. J Pediatr Urol 2008;4:221-8.
90. Nelson CP, Copp HL, Lai, J, et al. Is the availability of endoscopic treatment changing the initial management of vesicoureteral reflux? J Urol. 2009; 182: 1152–1157.
91. Stenberg AM et al. Lack of distant migration after injection of a 125iodine labeled dextranomer based implant in the rabbit bladder. J Urol 1997;158:1937-41.
92. Läckgren G et al. Long-term followup of children treated with dextranomer/hyaluronic acid copolymer for vesicoureteral reflux. J Urol 2001;166:1887-92.
93. Kirsch AJ et al. Minimally invasive treatment of vesicoureteral reflux with endoscopic injection of dextranomer/hyaluronic acid copolymer: the Children’s Hospital of Atlanta experience. J Urol 2003;170:211-5.
94. Dave S et al. Learning from the learning curve: factors associated with successful endoscopic correction of vesicoureteral reflux using dextranomer/hyaluronic acid copolymer. J Urol 2008;180:1594-1600.
95. Kirsch AJ et al. The modified STING procedure to correct vesicoureteral reflux: improved results with submucosal implantation within the intramural ureter. J Urol 2004;171:2413-6.
96. McMann LP et al. Long-term preservation of dextranomer/hylronic acid copolymer implants after endoscopic treatmet of vesicoureteral reflux in children: a sonographic volumetric analysis. J Urol 2007;177:316-20.
97. Elder J et al. Endoscopic therapy for vesicoureteral reflux: a meta-analysis. I. Reflux resolution and urinary tract infection. J Urol 2006;175:716-22.
98. Chertin B et al. Endoscopic treatment of vesicoureteral reflux: 11 to 17 years of followup. J Urol 2002;167:1443-5.
99. Haferkamp A et al. Failure of subureteral bovine collagen injection for the endoscopic treatment of primary vesicoureteral reflux in long-term follow-up. Urology 2000;55:759-63.
100. Capozza N et al. Dextranomer/hyaluronic acid copolymer implantation for vesico-ureteral reflux: a randomized comparison with antibiotic prophylaxis. J Pediatr 2002;140:230-4.
101. Lee EK et al. Long-term followup of dextranomer/hyaluronic acid injectin for vesicoureteral reflux: late failure warrants continued followup. J Urol 2009;181:1869-75.
102. Nepple KG et al. Rare variant of bladder exstrophy associated with urethral, bladder, and colonic duplication. Urology 2009;69:928.e1-3.
103. Knudson MJ et al. Calcification of glutaraldehyde cross-linked collagen in bladder neck injections in children with incontinence: a long-term complication. J Urol 2006;176:1143-6.
104. Gargollo PC et al. Mound calcification after endoscopic treatment of vesicoureteral reflux with autologous chondrocytes--a normal variant of mound appearance? J Urol 2009;181:2702-8.
105. Noe HN. Calcification in a Deflux bleb thought to be a ureteral calculus in a child. J Pediatr Urol 2008;4:88-9.
106. Vandersteen DR et al. Postoperative ureteral obstruction after subureteral injection of dextranomer/hyaluronic acid copolymer. J Urol 2006;176:1593-5.
107. Austin JC et al. Vesicoureteral reflux: surgical approaches. Urol Clin North Am 2004;31:543-57.
108. Sprunger JK et al. Can standard open pediatric urological procedures be performed on an outpatient basis? J Urol 2001;166:1062-4.
109. Marotte JB et al. Extravesical ureteral reimplantations for the correction of primary reflux can be done as outpatient procedures. J Uro 2001;165:2228-31.
110. Palmer JS. Bilateral extravesical ureteral reimplantation in toilet-trained children: short-stay procedure without urinary retention. Urology 2009;73:285-8.
111. Hayn MH et al. Minimally invasive treatment of vesicoureteral reflux. Urol Clin North Am 2008;35:477-88.
112. Barrieras D et al. Are postoperative studies justified after extravesical ureteral reimplantation? J Urol 2000;164:1064-6.
113. Bisignani G et al. Voiding cystourethrography after uncomplicated ureteral reimplantation in children: is it necessary? J Urol 1997;158:1229-31.
114. El-Ghoneimi A et al. Cystography after the Cohen ureterovesical reimplantation: is it necessary at a training center? J Urol 1999;162:1201-2.
115. Rolleston GL et al. Intrarenal reflux and the scarred kidney. Arch Dis Child 1974;49:531-9.
116. Austin JC. Treatment of vesicoureteral reflux after puberty. Adv Urology 2008;590185.
Figure Legends
Figure 1. International Reflux Grading System.
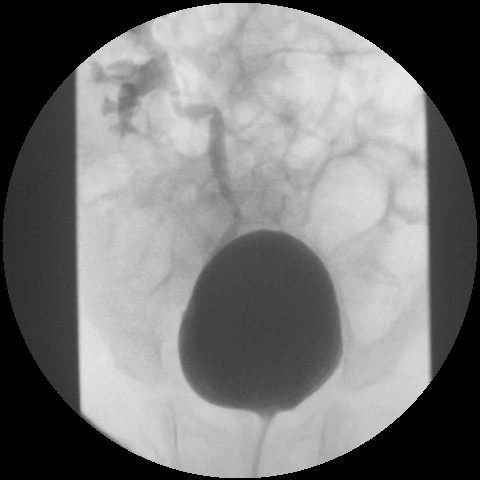
Figure 2. Grade III VUR.
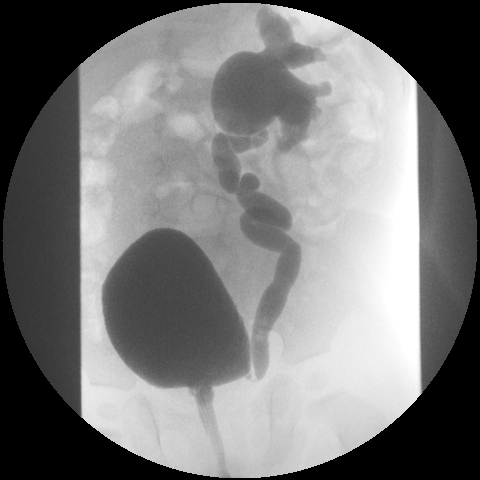
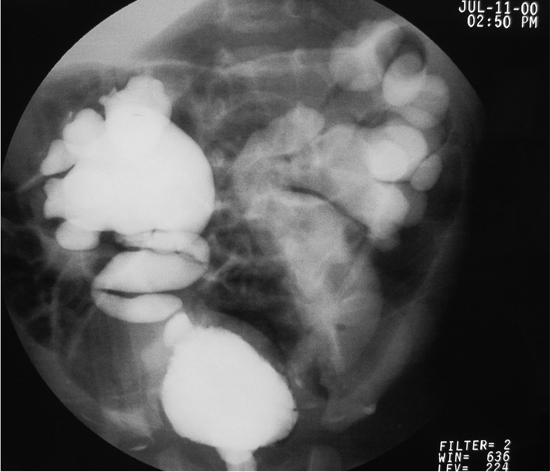
Figure 3. Grade IV VUR.
Figure 4. Grade IV VUR.
Figures 5A, 5B. Nuclear cystometrogram demonstrating onset of left VUR at 14 cm H2O.
Figure 6. Acute pyelonephritis demonstrating photopenic defects in left kidney
Figure 7. Intravesical ureteral implant.
Figure 8. Neural network incorporating predictive factors for resolution of reflux.

