Introduction
Posterior urethral valves (PUV) remains the most common congenital cause of bladder outflow obstruction in male neonates. The majority of cases are suspected prenatally and referred to specialist centres at birth. Simultaneously, the threshold for investigating male infants with urinary tract infections has been reduced and therefore early diagnosis is usually the norm. The spectrum of renal dysfunction and subsequent functional outcomes vary widely in children with this condition. PUV and its consequences, including renal dysplasia, upper tract dilatation, vesico-ureteric reflux, urinary tract infection and bladder dysfunction, accounts for 25 – 30 % of pediatric renal transplantations in the UK (UK Transplant Registry).
This chapter aims to provide an overview on posterior urethral valves.
Embryology
The embryological abnormality giving rise to posterior urethral valves is thought to represent either an anomalous insertion of the mesonephric duct into the uro-genital sinus, preventing normal migration of these ducts and their anterior fusion or a consequence of an abnormality of the cloacal membrane. Early classification of PUV was done by Hugh Hampton Young in 1919, which described types I-III based on post-mortem dissection studies. Later studies suggested a more uniform appearance to the obstructing posterior urethral membrane and the prospective assessment of non-instrumented urethras by Dewan et al1, found similar appearances in all cases studied. Their endoscopic appraisal revealed the membrane to attach posteriorly, just distal to the verumontanum. The membrane extended anteriorly and obliquely beyond the external sphincter with a variable sized aperture located within it, at the level of the verumontanum and they described the membrane as congenital obstructing posterior urethral membrane (COPUM).
Incidence and Genetic Aspects
The incidence of posterior urethral valves remains relatively constant, at 1 in 5000 live male births. There have been no established genetic bases, or potential environmental or maternal factors identified for this anomaly. Affected neonates do not tend to have any other system disorder other than the associated renal dysfunction; however, a higher incidence of cryptorchidism compared to the normal population has been noted.2 PUV is not included within the range of urinary tract anomalies associated with VATER or VACTERL groups of children, although a few cases of PUV in association with ano-rectal malformation have been reported.
Prenatal Diagnosis
The number of cases of PUV diagnosed prenatally has increased with the increased utilisation and sensitivity of prenatal anomaly scanning. In the UK, pregnant women are scanned on at least two occasions during pregnancy. The initial scan is performed around 10–12 weeks gestation and serves as a dating scan. A second scan is arranged around 20 weeks gestation and this is a detailed anomaly scan. Approximately ½-2/3 of boys with PUV will be suspected prenatally. Prenatal findings on ultrasonography in suspected cases may include a thick walled bladder, the ‘keyhole’ sign with a dilated bladder and posterior urethra, unilateral or bilateral hydroureteronephrosis, echobright kidneys and oligohydramnios. The differential diagnosis includes prune belly syndrome, urethral atresia, bilateral vesico-ureteric reflux and, less frequently, the megacystis-microcolon intestinal hypoperistalsis disorder. Early prenatal diagnosis (before 24 weeks gestation), ultrasound evidence of echo-bright renal parenchyma and reduced amniotic fluid volume have been identified as predictors of a poor prognosis.
Prenatal Intervention
Prenatal intervention for lower urinary tract obstruction has been practised for many years with current options being serial vesicocentesis or vesico-amniotic shunting. The rationale is that early decompression of the fetal renal tract will allow improved survival with preservation of renal function, a reduction in the respiratory compromise and limb abnormalities seen in association with severe oligohydramnios. However, there remains no clinical consensus about the efficacy and use of prenatal intervention.
Experimental studies have been successful in establishing the link between urine outflow impairment and renal dysplasia. Similarly, the ability to reverse these changes on removal of obstruction has also been independently described by Kitagawa3 and Harrison.4 Lung hypoplasia is seen only in association with oligohydramnios. This and similar experimental evidence has led researchers’ attempts to identify the fetuses who may benefit from prenatal intervention. Further refinement in the identification of the fetus who may benefit from such intervention has been attempted by analysis of fetal urine, urine osmolality, sodium and calcium concentration, beta2- microglobulin, and other markers. At the present time, there is no single marker or combination of fetal urine markers that is reliable. Serum beta2- microglobulin can be increased in fetuses with urinary tract anomalies, including PUV, but when levels in fetuses with lower urinary tract obstruction were analysed in the context of pre- and post-vesico-amniotic shunting, this parameter did not reliably predict post-natal renal function.5
The systematic review and meta-analysis by Clark et al in 2003 aimed to show the effect of prenatal bladder drainage on perinatal survival in 342 fetuses with lower urinary tract obstruction.6 Their review was hampered by a lack of high quality evidence, variable assessment criteria and reporting of outcome measures with the data examined being from case series (n=147) or controlled studies (n=195). No randomised controlled study was available for review at that time and this remains the case almost 10 years later. The review concluded that overall perinatal survival was suggested to improve (OR 8.1; 95%CI 1.2, 52.9; p=0.3) in those fetuses predicted to have a poor prognosis but not in those fetuses whose predicted outcome was favourable. In the survivors, the number with normal renal function was no different between those who did or did not undergo prenatal intervention.
Gestational age at diagnosis (<24 weeks) and oligohydramnios have been found to be significant predictors of a worse renal outcome.7 However, prenatal diagnosis, per se, has not led to improved long-term outcomes.
More recently, an attempt was made to address the question of benefit of prenatal intervention in the form of vesico-amniotic shunting in suspected bladder outflow obstruction. The PLUTO trial was set up as a pan-European multicentre trial.8 Entry into this trial was based on the uncertainty principle. In the event of doubt of the benefit of intervention, patients were randomised to vesico-amniotic shunting or no treatment. Outcome measures included postnatal mortality, renal function assessed by serum creatinine at age 1 year and any associated morbidity or complications of the procedure. The trial ran for 5 years but was unable to recruit the required number of patients and is now closed. Unfortunately, due to the small number of patients recruited it will not answer the lingering question of benefit of prenatal intervention in suspected bladder outflow obstruction.
Neonatal Management
For those neonates suspected of having PUV prenatally, with no concerns about amniotic fluid volume, the delivery may take place outside of specialist paediatric urology centres. The priority is to drain the bladder and a size 6 Fr urethral catheter should be placed and antibiotic prophylaxis started, e.g., trimethoprim 2mg/kg. In those neonates where it is not possible to catheterise urethrally, usually due to either non-availability of a 6 Fr catheter or a very high bladder neck that precludes successful catheterisation, a supra-pubic catheter becomes necessary. Following successful bladder drainage, close attention should be paid to urine output and electrolyte measurements as these patients may develop polyuria and/or abnormalities of sodium, potassium and bicarbonate as a result of excessive renal losses secondary to post-obstructive diuresis. Creatinine should be monitored on a daily basis until a nadir creatinine level is achieved. For patients with significant renal dysfunction and electrolyte disturbance, a nephrologist colleague should be involved from an early stage to optimise renal function and homeostasis.
In boys born with a history of oligohydramnios and significant pulmonary hypoplasia the priority of management is respiratory support and bladder drainage. Either a urethral or supra-pubic catheter is sufficient to optimise urinary drainage whilst awaiting homeostasis and respiratory stability.
Neonates and infants who were not suspected to have PUV prenatally may present with urosepsis, obstructive voiding symptoms, a distended bladder or palpable kidneys and the principles of management are the same. While poor urinary stream is suggestive of bladder outflow obstruction, the observation of a normal urinary stream does not exclude the diagnosis.
Diagnosis in the Neonatal Period
Once the neonate had been stabilised the diagnosis must be confirmed. Renal ultrasound scan (USS) will give information on the size and quality of the renal parenchyma, degree of hydroureteronephrosis and assessment of bladder wall thickness. It may also show a dilated posterior urethra.
Micturating cystouretrogram (MCUG) will confirm the diagnosis and must include catheter-out views to demonstrate the calibre of the posterior urethra (Figure 1)
MCUG demonstrating dilated posterior urethra, open bladder neck, irregular bladder and bilateral vesico-ureteric reflux.
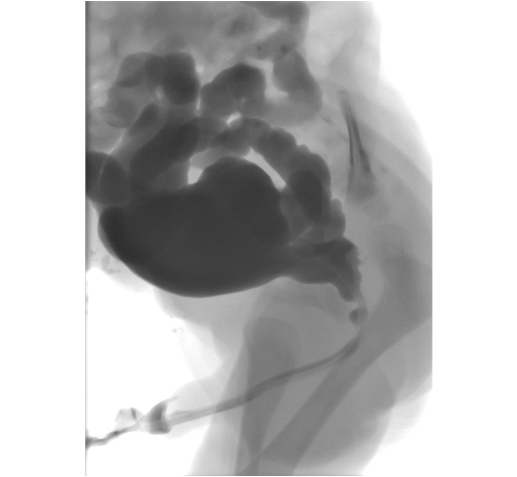
. Additional information obtained from the MCUG includes bladder size, bladder irregularity from thickening, trabeculation and diverticula formation, vesico-ureteric reflux (VUR) and the configuration of the bladder neck. Approximately 50% of neonates with PUV will have VUR. If the VUR has been high grade and unilateral, a “pop-off” may have allowed selective dissipation of back pressure, resulting from urethral obstruction.
The VURD (valves unilateral reflux dysplasia) syndrome described by Hoover and Duckett9 results in very poor or non-function of the kidney on the refluxing side with a relative sparing of renal function on the contralateral, non-refluxing side. These authors also postulated that this mechanism of ‘pop-off’ results in long-term normal renal function, as the contralateral kidney is spared and normal. This hypothesis was subsequently challenged by Cuckow et al who showed with serum creatinine and GFR measurements that renal function was impaired in cases with VURD, implying that the protection offered by the ‘pop-off’ mechanism was not complete.10
Primary Valve Resection
The procedure of choice for PUV is primary valve ablation which is performed once the baby is stable from a medical point of view. At induction of anaesthesia a dose of intravenous antibiotic is given, e.g., co-amoxiclav 30mg/kg. The baby is placed in the lithotomy position and a diagnostic cystoscopy using 0° 6/8 Fr neonatal cystoscope performed. The posterior urethra should be carefully inspected and the valve configuration noted. The configuration of the bladder neck and appearances of the bladder and ureteric orifices should also be noted. The 11 Fr resectoscope is assembled with either the cold/sickle blade or bugbee electrode and valve resection is typically performed at the 5, 7 and 12 o’clock positions (Figure 2).
Endoscopic view of PUV with the sites for valve incision marked.
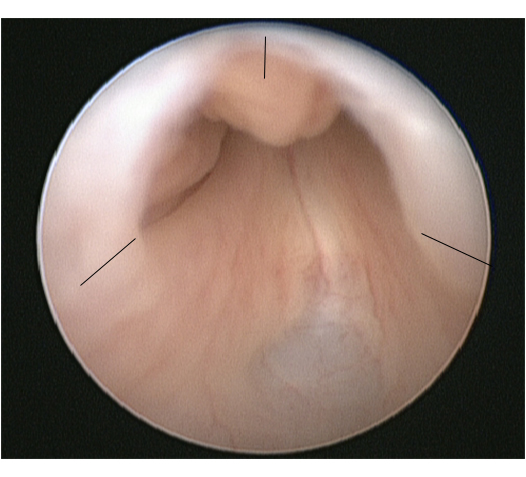
A urethral catheter is placed at the end of the procedure and removed 24-48 hours later. The creatinine should be checked following catheter removal and the nappies weighed to document urine output. Antibiotic prophylaxis and renal supplements should continue until further review.
The complications of primary valve ablation include bleeding, incomplete valve resection, urethral stricture or inadvertent damage to the external sphincter. The choice of technique is dictated by the instruments available, calibre of the neonatal urethra and the size and maturity of the baby.
For preterm or very small neonates, endoscopic resection is delayed due to the difficulties of the small calibre of the urethra and potential complications of using the relatively larger instruments. These children may be managed with a urethral or supra-pubic catheter until such time as they are big enough for the procedure. As a guide, a body weight of 2.5 kg should allow safe and accurate PUV resection with standard endoscopic instruments.
For some small neonates there may be repeated issues with urethral or suprapubic catheters falling out, becoming blocked or dislodged or not draining adequately and in these circumstances formation of a vesicostomy may be considered the safest option to ensure adequate and continuous bladder drainage.
It is our recommendation that all boys have a follow-up cystoscopy within 3 months of the primary procedure to ensure completeness of valve ablation. This is due to the poor correlation of repeat MCUG in diagnosing residual valve leaflets and cystoscopic appraisal of residual valve leaflets requiring further resection; positive predictive valve 56%.11 However, some centres would choose to perform a repeat MCUG at this stage and proceed to cystoscopy only if the MCUG suggests persisting urethral obstruction. A further advantage of proceeding directly to check cystoscopy is that it provides an opportunity to perform circumcision which has been shown to reduce the rate of urinary tract infection by at least 83% in boys with posterior urethral valves.12
Presentation, Diagnosis and Management in the Older Child
A smaller number of patients with PUV will not present as neonates or infants but attend clinics in childhood or early adolescence with symptoms of diurnal enuresis, dribbling or poor urine stream, infection or less commonly hematuria. A careful voiding history may suggest the diagnosis and non-invasive studies, such as flow rates and measurement of post-void residual volumes, may also point to the abnormality. Urethral catheterisation in the older child is invasive and fraught with difficulties; therefore, our preference is to omit the MCUG and instead, proceed directly to a diagnostic cystoscopy and primary valve ablation if the diagnosis is confirmed. Follow-up after successful complete valve ablation is as outlined below with a greater suspicion of persistent bladder dysfunction in those presenting late.
Follow-up
Each institution will have a different follow-up regimen for children with posterior urethral valves. However, the principles of follow-up are universal with the objectives being to maximise renal function, minimise urinary infections and renal scarring, assessment and management of voiding dysfunction with the aim of attaining total urinary continence and eventually arrange transition to adolescent and adult services. The involvement of pediatric nephrology is imperative to maximise outcomes and to ensure the correct medical management of these children (Table 1).
|
Creat &
Electrolytes |
FBC |
Corrected
GFR |
US |
MCUG |
MAG3 |
Cystoscopy/
valve ablation |
MSU |
BFA |
Video-
Urodynamics
via SPC |
Birth |
√ |
√ |
|
√ |
√ |
|
√ |
|
|
|
3 months |
√ |
√ |
|
√ |
|
√ |
+/- circumcision |
√ |
|
|
1 year |
√ |
√ |
√ |
√ |
|
|
|
√ |
√ |
|
2 years |
√ |
√ |
|
√ |
|
√ |
|
√ |
√ |
|
3 years |
√ |
√ |
√ |
√ |
|
|
|
√ |
√ |
|
4 years |
√ |
√ |
|
√ |
|
√ |
|
√ |
√ |
√ |
5 years |
√ |
√ |
√ |
√ |
|
|
|
√ |
√ |
|
6 years |
√ |
√ |
|
√ |
|
√ |
|
√ |
√ |
|
7 years |
√ |
√ |
|
√ |
|
|
|
√ |
√ |
|
8 years |
√ |
√ |
|
√ |
|
√ |
|
√ |
√ |
|
9 years |
√ |
√ |
|
√ |
|
|
|
√ |
√ |
|
10 years |
√ |
√ |
√ |
√ |
|
√ |
|
√ |
√ |
|
11-14 yr
Annual |
√ |
√ |
|
√ |
|
|
|
√ |
√ |
|
>15 yr |
√ |
√ |
√ |
√ |
|
+/- |
|
√ |
√ |
Adolescent
referral |
Table 1- Example of PUV patient follow-up protocol used at Great Ormond Street Hospital, UK
FBC full blood count
GFR glomerular filtration rate
US renal tract ultrasound
MCUG micturating cystourethrogram
MSU midstream specimen of urine, to include test for proteinuria
BFA bladder function assessment.
Age related; in infants nappy alarms and post void residual measurements; in older children frequency-volume charts, flow studies and post void residual measurements
SPC supra-pubic catheter
Early Urinary Diversion
There is a small group of boys in whom the standard measures outlined above do not produce the desired outcome and their renal function remains fragile or deteriorates, they suffer recurrent urinary tract infections or there is significant deterioration in the appearance of the upper tracts or bladder emptying is incomplete on serial ultrasounds. The aim in these children is to maximise their renal potential and to delay or avoid renal replacement. Early urinary diversion, either by vesicostomy or bilateral ureterostomy or pyelostomy, aims to protect the upper tracts and minimise the risks of infection.
Early diversion with the Sober ureterostomy at the time of primary valve ablation, or shortly afterwards by Ghanem and Nijman, was reported as allowing for an improvement in serum creatinine in 21 patients.13 Tietjen et al carried out supra-vesical diversions in 26 patients and reported that this did not offer an improvement in the outcome of renal function in the long-term.14 However, Lopez-Pereira at al found that in 24 boys with renal impairment at the time of diagnosis treated with pyelo-ureterostomy, the glomerular filtration rate at 1 year of age was improved in the diverted group but by age 5 years this improvement was no longer evident.15
A recent development is the use of a refluxing ureterostomy as a form of urinary diversion. This is an elegant technique; it allows bladder cycling to continue and at the same time dissipates the high storage and voiding pressures that are present in the valve bladder at this age. The primary requirement is a freely refluxing ureter and the procedure is carried out through a low groin skin crease incision. The ureter is identified and isolated extra-vesically in the retroperitoneum. A loop refluxing ureterostomy is then fashioned and will drain into the nappy. Unlike the end ureterostomy, reversal is relatively easy. Once the procedure has served its purpose or stops refluxing, the ureterostomy is simply closed and the ureter returned to the retroperitoneal space. The refluxing ureterostomy is particularly useful in boys with fragile renal function, as it will minimise the potential detrimental effects of the high-pressure bladder that is present in the early period following valve ablation. This is critical in the management of these neonates as renal replacement in infancy is extremely precarious and difficult to maintain.
There have been legitimate concerns that bypassing the bladder or having a continuously empty bladder may negatively impact future bladder function. Additionally, there is a lack of convincing evidence that urinary diversion improves long-term renal function.
When comparing primary valve ablation with primary vesicostomy, Godbole et al found no significant difference in serum creatinine and glomerular filtration rate (GFR) at 1 year of age between the two groups.16 The group who had vesicostomy formation as their primary procedure had been diverted for a median time of 18 months and 7 boys who had subsequent urodynamics demonstrated a normal bladder capacity. Jaureguizar et al compared bladder function outcomes in boys treated with supravesical diversion with primary valve ablation alone.17 The mean time for which supravesical diversion was present was 13 months and all were diverted in the first 2 months of life. They carried out invasive urodynamic studies at age 9–10 years and found very similar proportions of normal, poorly compliant, unstable and failing bladders in both groups.
More favourable bladder outcomes, improved capacity, lower detrusor end-fill pressure at expected bladder capacity, and less frequent detrusor instability have been demonstrated in 8 patients managed with primary valve ablation compared to 11 boys managed with high and low loop ureterostomy.18 The urodynamic studies were performed in these boys at a minimal mean age of 11 years and the duration of diversion was a mean of 57 months.
Thus, the place of early urinary diversion in the management of boys with PUV is limited. It has the potential to improve renal function in the short-term which is very important in boys with fragile kidney function and can defer renal replacement to a later stage. There is no convincing evidence to support its role as a way of improving long-term renal function and the jury is out on its effect on long-term bladder function. Therefore, urinary diversion must be considered in selected cases with clear goals and endpoints in mind, and has an important place in the management of boys with PUV.
Long-term Outcomes: the Upper Urinary Tract
Urine production begins at 8-9 weeks gestation and the collecting system is formed by the end of the first trimester. The fetal bladder can be seen soon after this on prenatal ultrasound. The presence of bladder outflow obstruction transmits raised intraluminal pressure to the developing renal parenchyma and once a critical level is reached causes apoptosis, abnormal cellular differentiation and glomerular changes that occur within the kidney. These early changes dictate the functional renal potential in later life. In cases where the obstruction is less severe or declares itself later in pregnancy, the effects of obstruction tend to be more on the bladder and renal effects are limited to dilatation of the collecting system with minimal disruption of normal nephrogenesis. An alternative or perhaps supplementary theory to explain the renal dysplasia seen in conjunction with posterior urethral valves relates to abnormal position of the ureteric bud and implantation into the metanephric blastema.19 Renal dysfunction thus appears to be the result of varying degrees of inherent dysplasia and the effects of bladder outflow obstruction. In post-natal life these effects can be further exaggerated by urinary tract infections, VUR and bladder dysfunction
When there is a significant loss of nephrons, hyperfiltration of existing functional nephrons occurs via vasodilatation of the afferent arterioles. The resultant glomerular capillary hypertension allows for normal renal function to be temporarily maintained. Over time this compensatory mechanism decompensates, glomerulosclerosis ensues, with resulting proteinuria, hypertension and a reduced glomerular filtration rate. Damage to the distal nephron impairs the concentrating ability of the kidney resulting in polyuria and polydipsia (nephrogenic diabetes insipidus), which can worsen bladder function and thereby further risk upper tract function.
The paper by Parkhouse et al is often quoted and describes the long-term outcomes of 98 boys diagnosed with posterior urethral valves presenting between 1966-1975, before the era of antenatal diagnosis.20 The follow-up period of this study ranged from 11 to 22 years. 66% of the cohort presented in the first year of life and half of the cohort of patients were managed with primary valve ablation, the remainder being diverted. 32% (31 patients) had a ‘bad long-term renal outcome;’ 10 patients died of acute renal failure, 15 developed end-stage renal disease and 6 had established chronic renal impairment over the follow-up period. Presentation under the age of one year (p<0.05), presence of bilateral vesico-ureteric reflux (p<0.0010), a plasma urea on initial discharge of >10 mmol/litre, and at age 5 years, presence of proteinuria (p<0.001) along with persistent urinary incontinence were all associated with a poor renal outcome.
More contemporary series report rather similar results. Kousidis et al reported a series of 42 patients with pre and postnatally diagnosed posterior urethral valves between 1984-1996.21 The follow-up period was 10-23 years. There were 3 (7%) deaths (2 in the neonatal period and 1 patient age 3 years following unsuccessful transplantation), 11 patients (26 %) had progressed to end-stage renal failure, one of these occurring only in the late teenage years.
In a group of 65 PUV patients presenting during 1987-2004 and followed over a shorter time period (range 1-14.3 years; median follow-up 6.8 years), Sarhan et al found a 17% overall rate of renal impairment of whom 9% had progressed to ESRF.7 Early gestational age at diagnosis (<24 weeks; p=0.003) and pre-natal oligohydramnios or anhydramnios (p=0.02) was associated with a poor outcome. There were no deaths in this group but during the same study period there were 14 fetuses with a prenatal diagnosis in which the pregnancy was terminated. Fetal urine analysis had been done in all cases and post mortem findings confirmed severe renal histological changes. This suggests that the overall incidence of renal failure, if taking into account the fetuses that were terminated, is similar to the other studies above.
DeFoor’s larger group of 119 PUV patients followed for a similar length of time, (range 3-24 years; mean follow-up 7.2 years), found 13% to have developed end stage renal failure by a median age of 8.2 years (range 7 days- 17.5 years).22 In this study, bladder dysfunction, necessitating clean intermittent catheterisation and/or use of anti-cholinergics, was associated with an increased risk of ESRF. Nadir serum creatinine > 1.0 mg/dl was also associated with an increased risk of ESRF (OR 71, CI 10-482) and this has been borne out in several previous and subsequent studies where serum creatinine <1.0 mg/dl at 1 year of age affords a good long-term renal outcome.
Heikkila et al have evaluated a larger cohort of patients (n=193) with posterior urethral valves over a much longer period of time (range 6-69 years; median follow-up 31 years).23 The rate of progression to end stage renal failure was 22.8%, with 68% developing ESRF before the age of 17 years and the remainder after the age of 17 years. The deterioration in renal function around the time of puberty in children with dysplastic kidneys is well recognised24 and a likely explanation is that it represents changes within the kidney in response to growth, increased body mass and increased blood pressure. However, this late deterioration in renal function after puberty highlighted by this paper highlights the need for follow-up of these patients into adulthood and beyond.
Vesico-ureteric Reflux: At presentation, approximately 50% of patients have vesico-ureteric reflux (VUR) on the initial MCUG. This is believed to be secondary to the bladder outflow obstruction and co-existent bladder dysfunction. Approximately 15% of patients will demonstrate unilateral high grade VUR with non-function of the ipsilateral kidney. Following valve ablation the severity of VUR may decrease or resolve completely in 25-50% of cases, and this improvement is more likely in those presenting as neonates or during infancy. Persistent VUR, especially high grade and bilateral, after successful valve ablation is associated with poor long-term renal outcome and is one of the poor prognostic indicators identified by Parkhouse et al.20 There is no longer the trend to perform anti-reflux procedures in boys with PUV, as failure rates associated with this approach are high. In cases where the grade of reflux is high and associated with poor function in the ipsilateral kidney, the capacious ureter has been used to augment the bladder in association with removal of the non-functioning kidney. Other innovative strategies include creating a refluxing ureterostomy as previously discussed and occasionally the distal ureter has been used as a Mitrofanoff channel with or without an associated anti-reflux procedure. Persistent higher grades of reflux are a potential risk factor for recurrent urinary infection and can impact negatively on transplanted kidneys and are therefore usually treated surgically prior to renal transplantation.
Hydro-ureteronephrosis: The majority of neonates presenting with posterior urethral valves will demonstrate bilateral hydro-ureteronephrosis. This is often seen to improve with initial catheterisation but may temporarily worsen due to functional obstruction at the level of the VUJ by the thickened bladder wall that collapses, pinching off the ureteric orifices following catheterisation and decompression. It is important to recognise this phenomenon and occasionally this can result in anuria that lasts for 24-48 hours. The obstruction spontaneously resolves within 48-72 hours and is usually followed by post-obstructive diuresis. It is important to keep a steady nerve and resist the temptation to try and decompress the upper tracts with either internal JJ stenting or placement of nephrostomies. After primary valve ablation one should anticipate the degree of upper tract dilatation to gradually improve or resolve altogether.25 This improvement will be influenced by ongoing VUR, bladder dysfunction, polyuria and abnormal ureteric motility and all these factors must be accounted for when assessing outcomes following valve ablation. Ureteric re-implantation with or without tapering is no longer performed in the context of worsening hydro-ureteronephrosis in cases of PUV as the role of the bladder and its associated dysfunction and renal tubular dysfunction with resultant polyuria have become increasingly recognised with improved understanding of the pathophysiology of bladder outflow obstruction.
Long-term Outcomes: the Lower Urinary Tract
The underlying aetiology of bladder behaviour in posterior urethral valves remains a topic of much debate. The two main culprits implicated in the aetiology are that the abnormal bladder is the result of in-utero changes in response to outflow obstruction and/or that the bladder dysfunction is the result of urinary diversion. The jury is out on the second theory, as currently, the majority of cases are not diverted although the incidence of bladder dysfunction remains high. In addition Godbole et al16 have shown that diversion is not necessarily detrimental to outcome of bladder function and may even improve it.
Histologically, the obstructed bladder in fetal animal models demonstrates an increase in smooth muscle mass, an increase in the number of muscarinic receptors and increased collagen deposition. Levin et al have shown the bladder to pass through three phases in response to obstruction.26 Researchers have also demonstrated reversibility in some of these changes with removal of obstruction although recovery may be partial depending on the timing of reversal and the degree of irreversible damage that has already occurred. The early presentation that results from prenatal diagnosis allows for prompt treatment and therefore it would be reasonable to expect improved outcomes for bladder function in the current population of boys with prenatally suspected bladder outflow obstruction.
The term ‘valve bladder syndrome’ was introduced as a concept in the 1980s to encompass the abnormal voiding patterns and symptoms of voiding dysfunction, the persistent thick walled bladder, incomplete bladder emptying and associated upper tract dilatation seen in many boys with posterior urethral valves. Peters et al, in their cohort of 41 boys who were evaluated mainly for persistent urinary incontinence and ‘valve bladder syndrome,’ found three dominant urodynamic patterns on invasive testing; the hyper-reflexic bladder, hypo-compliant or acontractile bladder with some overlap between these patterns.27
Over the last 30 years clinicians have begun to recognise and understand the importance of bladder dysfunction and its impact outcome of both urinary continence and renal function in boys with posterior urethral valves. Marked differences in the technique of urodynamics, classification of different patterns and timing of examinations are seen when reviewing the literature on this topic.
Holmdahl et al followed 12 boys with PUV under the age of 15 years. Instability during urodynamic filling was observed in 2/3 of patients at 5 years of age but by puberty this had reduced.28 Bladder capacity at age 5 years appeared to be normal but after puberty capacity was shown to be approximately twice expected capacity. The urodynamic pattern changed over time with instability decreasing in favour of an over distended pattern with increasing age. This change in bladder behaviour was also shown by De Gennaro et al, where 71% of 48 patients studied had abnormal urodynamic studies between ages 10 months-15 years.29 In the age group <8 years, 44% had hypercontractile and 31% hypocontractile bladders and patients >8 years demonstrated 28 % hypercontractile and 50% hypocontractile urodynamic patterns. Misseri et al demonstrated a lower rate (5.9%) of myogenic failure (acontractile or unable to generate a sustained detrusor contraction for adequate bladder emptying) in their retrospective study of 51 boys and concluded that the detrusor failure was due to co-existing anti-cholinergic treatment in their cohort of cases.30
Bladder dysfunction is key to long term renal function outcomes as illustrated in the long term follow-up study by Parkhouse et al, where the finding of incontinence at 5 years in 44% of patients, defined as not being dry during the day, was associated with a poor renal outcome in 46% of this group (p<0.001).20 In Ansari’s group of 227 boys with posterior urethral valves, they showed an overall 30% risk of developing chronic kidney disease with 10% progressing to end stage renal failure.31 Severe bladder dysfunction, defined as low compliance with end filling pressure >40 cm of H20 or post-void residual volume >30% or underactive detrusor or the need for CIC, was more prevalent in the patients progressing to ESRF (p<0.0001). Mazen’s study of 116 patients with PUV followed for a mean of 10.3 years (range 18 months-22 years) found 42% of patients had ESRF or had been transplanted. Urodynamic abnormalities were observed in 80% of the overall patient group and bladder instability and poor compliance were correlated with a poor renal functional outcome (p=0.04).32
Bladder function continues to evolve through adolescence and effective bladder management must continue into adulthood. Prostatic growth alters the dynamics of bladder outflow and the changing pattern of bladder dynamics seen in early childhood continues to change through adolescence with enlarged bladder capacity and large post-void residual volumes being most prevalent. Some young men may have to initiate clean intermittent catheterisation to address this irreversible deterioration in bladder function.
Renal Transplantation
With a significant number of boys with PUV progressing to end-stage renal failure, the importance of a comprehensive bladder assessment as part of the pre-transplant work-up cannot be over-emphasised. A high pressure, poorly compliant, low capacity bladder may put at risk the transplanted kidney and graft loss becomes a real possibility. Five year graft survival rates in the PUV population have improved over the last 2 decades from 40% in the 1980s to near 70% in the 1990s.
For those patients who require surgical intervention to achieve a bladder which is considered a ‘safe’ receptacle for the transplant there are divided opinions as to whether this surgery should be performed before or after transplantation. Performing bladder augmentation with/without a catheterisable conduit prior to renal transplant allows post-operative healing without immuno-suppression but risks a ‘dry cystoplasty’ which must be managed by bladder cycling and lavages especially if there is no or minimal native urine production. Additional risk with this approach is that this major surgery may tip the fragile renal function over the edge and accelerate the need for replacement therapy and temporary dialysis in the pre-emptive transplant scenario. With pre-transplant bladder augmentation any additional procedures, including the transplantation itself, must not damage the vascular pedicle to the augmentation particularly when there is a short time interval between the procedures and extra care must be taken to ensure the safety of the reconstructed bladder. For those patients in whom augmentation cystoplasty is performed after renal transplantation it is important that immuno-suppression requirements have been stabilised and the improved renal function offers clear advantages. In this scenario the transplant ureter may be reimplanted into the native bladder or brought out as a cutaneous ureterostomy. In the context of posterior urethral valves and renal transplantation it is crucial that continued surveillance of bladder function continues after transplantation. Riley et al gives a comprehensive review of the different strategies of managing the bladder in the context of lower urinary tract dysfunction and renal transplantation.33
Fertility
There are few studies reporting on fertility and paternity outcomes in men with a history of posterior urethral valves. Persisting dilatation of the posterior urethra, damage to tissues around the verumontanum or secondary urethral strictures resulting from previous surgery will all influence the efficacy of ejaculation. Erectile dysfunction is seen more commonly in patients with chronic kidney disease and those on dialysis. In 9 patients with a history of PUV studied by Woodhouse34, the semen analysis was considered within the normal range whilst in 6 men who submitted semen for analysis to Lopez Pereira35 2 patients had abnormal forms or high percentage of immotile sperm.
Conclusion
- Posterior urethral valves remains the most common cause of neonatal bladder outflow obstruction in males.
- An increasing number of cases are diagnosed antenatally but prenatal intervention does not appear to confer a benefit in the long-term outcome of renal function.
- Primary valve ablation is the recommended treatment of choice with diversion being reserved for specific individual cases.
- A significant number of boys with PUV will develop chronic kidney disease and end stage renal failure.
- Structured follow-up aims to prevent upper tract deterioration, prevent urinary tract infection, maximise growth and allow surveillance for bladder dysfunction.
Posterior urethral valves
References
1. Dewan PA, Zappala SM, Ransley PG, Duffy PG. Endoscopic reappraisal of the morphology of congenital obstruction of the posterior urethra. J Urol 1992;70:439-444
2. Heikkila J, Taskinen S, Toppari J, Rintala R. Posterior urethral valves are often associated with crytorchidism and inguinal hernia. J Urol 2008;180(2):715-717
3. Kitagawa H, Pringle KC, Koike J, Zuccullo J et al. Vesicoamniotic shunt for complete urinary tract obstruction is partially effective. J Pediatr Surg 2006;41(2):394-402
4. Glick PL, Harrison MR, Adzick NS, Noall RA et al. Correction of congenital hydronephrosis in utero IV: in utero decompression prevents renal dysplasia. J Pediatr Surg 1984;19(6):649-657
5. Craparo F, Rustico M, Tassis B, Coviello et al. Fetal serum beta2-microglobulin before and after bladder shunting; a 2 step approach to evaluate fetuses with lower urinary tract obstruction. J Urol 2007;178(6):2576-2579
6. Clark TJ, Martin WL, Divakaran TG, Whittle MJ et al. Prenatal bladder drainage in the management of fetal lower urinary tract obstruction: a systematic review and meta-analysis. Obstet Gynecol 2003;102(2):367-382
7. Sarhan O, Zaccaria I, Macher M, Muller F et al. Long-term outcome of prenatally detected posterior urethral valves: a single centre study of 65 cases managed by primary valve ablation. J Urol 2008;179(1):307-312
8. PLUTO collaborative study group, Kilby M, Khan K, Morris K, Daniels J et al. PLUTO trial protocol: percutaneous shunting for lower urinary tract obstruction randomised controlled trial. BJOG 2007;114(7):904-905,e1-4
9. Hoover DL, Duckett JJ. Posterior urethral valves, unilateral reflux and renal dysplasia: a syndrome. J Urol 1982;128(5):994-997
10. Cuckow PM, Dineen MD, Risdon RA, Ransley PG et al. Longterm renal function in posterior urethral valves, unilateral reflux and renal dysplasia syndrome. J Urol 1997;158(3 pt 2):1004-1007
11. Smeulders N, Makin E, Desai D, Duffy P et al. The predictive value of a repeat micturating cystourethrogram for remnant leaflets after primary endoscopic ablation of posterior urethral valves. J Ped Urol 2011;7(2):203-208
12. Mukherjee S, Joshi A, Carroll D, Chandran H at al. What is the effect of circumcision on risk of urinary tract infection in boys with posterior urethral valves? J Ped Surg 2009;44:417-421
13. Ghanem MA, Nijman RJM. Long-term follow-up of bilateral high (Sober) urinary diversion in patients with posterior urethral valves and it’s effect on bladder function. J Urol 2005;173:1721-1724
14. Tietjen DN, Gloor JM, Husmann DA. Proximal urinary diversion in the management of posterior urethral valves: is it necessary? J Urol 1997;158:1008-1010
15. Lopez Pereira P, Espinosa L, Martinez Urrutina MJ, Lobato R et al. Posterior urethral valves: prognostic factors. BJU Int 2003;91:687-690
16. Godbole P, Wade A, Mushtaq I, Wilcox D. Vesicostomy vs. primary valve ablation of posterior urethral valves: Always a difference in outcome? J Ped Urol 2007;3:273-275
17. Jaureguizar E, Lopez Pereira P, Urrutina MJM, Espinosa L et al. Does neonatal pyeloureterostomy worsen bladder function in children with posterior urethral valves? J Urol 2000;164:1031-1034
18. Podesta M, Ruarte A, Garguilo C, Medel R et al. Bladder function associated with posterior urethral valves after primary valve ablation or proximal urinary diversion in children and adolescents. J Urol 2002;168:1830-1835
19. Henneberry MO, Stephens FD. Renal hypoplasia and dysplasia in infants with posterior urethral valves. J Urol 1980;123:912-915
20. Parkhouse HF, Barratt TM, Dillon MJ, Duffy PG at al. Long-term otcome of boys with posterior urethral valves. Br J Urol 1988;62:59-62
21. Kousidis G, Thomas DFM, Morgan H, Haider N et al. The long-term outcome of prenatally detected posterior urethral valves:10 to 23 year follow-up study. BJU Int 2008;102:1020-1024
22. DeFoor W, Clark C, Jackson E, Reddy P et al. Risk factors for end stage renal disease in children with posterior urethral valves. J Urol 2008;180:1705-1708
23. Heikkila J, Holmberg C, Kyllonen L, Rintala R at al. Long term risk of end stage renal disease in patients with posterior urethral valves. J Urol 2011;186:2392-2396
24. Celedon CG, Bitsori M, Tullus K. Progression of chronic renal failure in children with dysplastic kidneys. Pediatr Nephrol 2007;22:1014-1020
25. Farhat W, McLorie G, Capolicchio G, Khoury A et al. Outcomes of primary valve ablation versus upper tract diversion in patients with posterior urethral valves. Urology 2000;56(4):653-657
26. Levin R, Monson FC, Haugaard N, Buttyan R et al. Genetic and cellular characteristics of bladder outflow obstruction. Urol Clin North Am 1995;22(2):263-283
27. Peters CA, Bolkier M, Bauer SB, Hendren WH et al. The urodynamic consequences of posterior urethral valves. J Urol 1990;144(1):122-126
28. Holmdahl G, Sillen U, Hanson E, Hermansson G et al. Bladder dysfunction in boys with posterior urethral valves before and after puberty. J Urol 1996;155:694-698
29. De Gennaro M, Mosiello G, Capitanucci ML, Silveri M et al. Early detection of bladder dysfunction following posterior urethral valves ablation. Eur J Pediatr Surg 1996;6:163-165
30. Misseri R, Combs AJ, Hrowitz, Donohoe JM et al. Myogenic failure in posterior urethral valve disease: real or imagined? J Urol 2002;168:1844-1848
31. Ansari MS, Gulia A, Srivastava A, Kapoor R. Risk factors for progression to end-stage renal disease in children with posterior urethral valves. J Pediatr Urol 2010;6(3):261-264
32. Ghanem MA, Wolffenbuttel KP, de Vylder A, Nijman RJM. Long-term bladder dysfunction and renal function in boys with posterior urethral valves based on urodynamic findings. J Urol 2004;171:2409-2412
33. Riley P, Marks SD, Desai D, Mushtaq I et al. Challenges facing renal transplantation in pediatric patients with lower urinary tract dysfunction. Transplantation 2010;89(11):1299-1307
34. Woodhouse C, Reilly JM, Bahadur G. Sexual function and fertility in patients treated for posterior urethral valves. J Urol 1989;142(2 part 2):586-588
35. Lopez Pereira P, Miquel M, Martinez Urrutina MJ, Moreno JA et al. Long-term bladder function, fertility and sexual function in patients with posterior urethral valves treated in infancy. J Pediatr Urol 2011. Epub ahead of print.












