The sine hypospadias is characterized by a ventral curvature of the penile shaft and an orthotopic position of the meatus.
- The Megalomeatus is characterized by a coronal lying meatus adjacent to a non closed-glans with an open navicular fossa and a circular prepuce.
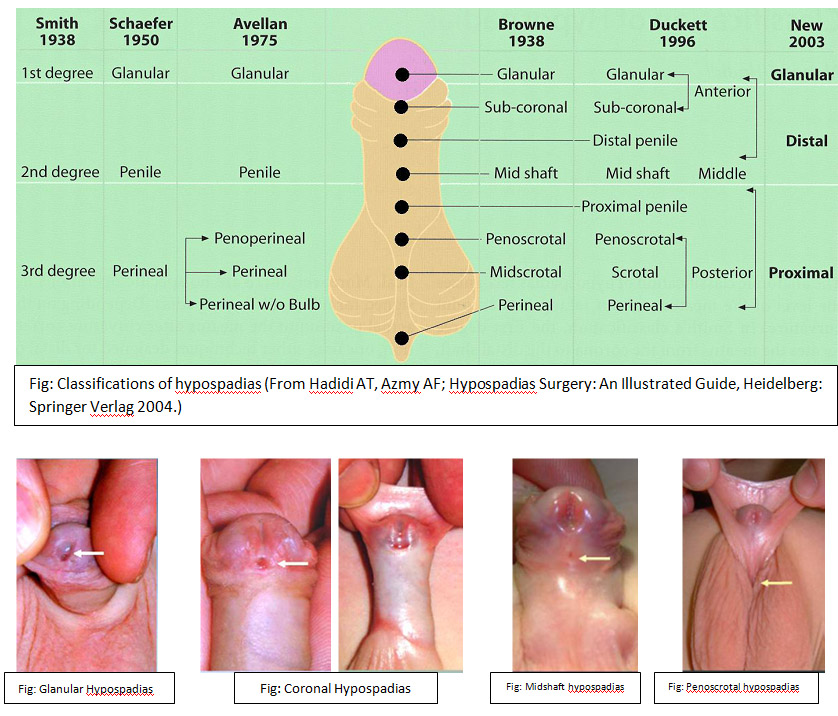
Classification
The most common classification was published by Duckett in 1996. He divided them into anterior (50%), middle (30%) and posterior (20%) hypospadias.
These are also classified according to the location of the meatus.
- The anterior form: glandular, coronal and distal penile.
- The middle form: "midshaft” and proximal penile.
- The posterior form: penoskrotal, scrotal and perineal.
Other classifications are listed in the following figure.
Frequency
The incidence of hypospadias has been reported to be anywhere between 0.4 to 8.2 cases per 1000 newborn boys. The large differences in frequency have their origin in geographical, genetic and environmental influences, as well as the variety of data collection. According to the "Centers for Disease Control" the highest probability of hypospadias is in the Caucasian population followed by African-Americans and then the Hispanic Americans.
The incidence of hypospadias is increasing in both Europe and North America but the reasons are not yet clearly understood. According to information from the "Birth Defects Monitoring Program”, reports show an increase in the incidence of 20.2 per 10,000 in 1970 to 39.7 per 10,000 children born in the U.S.A. This increase was independent of improvements in medical care and improved diagnostics. Similarly, according to Borer and Retik (1999), there has been an increase in the number of more severe hypospadias five fold compared with the more distal forms. This can be explained by the increase in the number of premature births, and the contact of the fetus with progesterone or other substances with estrogenic or anti-androgenic effect. On the other hand, there has not been an increase in the incidence in developing countries.
Etiology
The etiology of hypospadias is multifactorial. In 66-75% of hypospadias the cause remains unknown (Spindler et al. 2001). Somatic sexual development is associated with essential androgens. Therefore, it seems likely that defects in the androgen synthesis or its action during embryogenesis lead to hypospadias.
According to Spindler et al. (2001), hypospadias is also found in the most common disorder of sex development, the familial male pseudohermaphroditism. Zhu et al. (1998), and Geraudie and Ferreti (1998) demonstrated androgen metabolism failure, androgen receptor defects or genetic disorders in less than 5% of patients. Another cause is the ingestion of substances with estrogenic activity, such as insecticides, natural estrogens, organic products from the manufacture of plastics and pesticides, which are included in food. These are suspected to be the cause of increased incidence of hypospadias in developed countries. Semenza et al. showed in 1997, after animal models, that estrogens can lead to developmental delay and stop development of the penis.
A genetic predisposition is discussed as a cause of hypospadias. The probability to have a second child with hypospadias is 14%. If the father has hypospadias, the probability that one of his sons suffers from the same pathology is 8%. Similarly, hypospadias is present in many syndromes, such as Smith-Lemli-Opitz syndrome or Robinow syndrome. However, in these cases the exact cause is unknown
Risk Factors
There is an increased probability for the occurrence of hypospadias, under certain conditions
- Father with hypospadias
- Low birth weight
- Twin or triplet births
- Maternal iron supplements
- Smoking mothers
- Fathers with pesticide contact
- Pregnancy through artificial insemination or medical support
Embryology
The chromosomal sex is determined at fertilization by haploid sperm. If a Y chromosome is present, the indifferent gonad in stage 18 is differentiated into a testis and determines the gonadal sex. The formation of the somatic sex is determined by the existing gonad. Androgens are formed in the fetal testis. The Sertoli cells produce the Anti Müllerian Hormone (AMH) and the Leydig cells the testosterone. The distribution of these two hormones is responsible for the development of the male genitalia (Drews 2006). These hormones induce the extension of the genital tubercle into the penis. Growth in length is formed on both sides of the so-called urethral genital tubercle. Thus, the urethral folds form the lateral walls of the deep urethral groove. With the disappearance of the urogenital membrane a column is formed, which grows until the bottom of the genital tubercle, but falls short of the distal glans. Endodermal cells from the epithelial lining of the column form the urethral plate. Towards the end of the third month the urethral folds join above the urethral plate to form the penile urethra. The fusion area of the urethral folds migrates from the base to the tip of the penis. However, the tip of the penis is not reached.
By the end of the fourth month the distal portion of the urethra is formed, through the migration of the endoderm cells from the tip of the penis inward, forming a short epithelial cord, which grows on the existing urethra. This epithelial cord is channeled to form the definitive urethral meatus at the tip of the glans. The exception to this is the concept of endodermal differentiation, according to which the urothelium along the urethra comes from the urogenital sinus.
Therefore, the urethra is formed by the continuous growth of the urethral plate in the genital tubercle, followed by the ventral fusion of the urethra.
The edges of the urethral plate grow forward over the coronary sulcus and give rise to the foreskin, and a circular epithelial plate sinks between the folds and the glans.
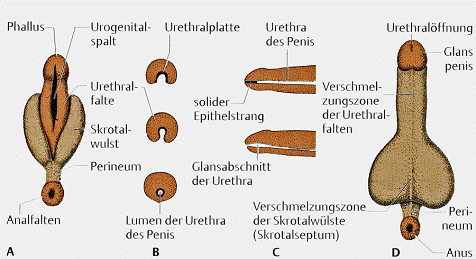
Fig: Development of the external genitalia in the male fetus
- A: 10 week old fetus. Between the urethral folds lies the deep urethral plate.
- B: Cross section of the phallus during the formation of the penile urethra. The urethral column is divided by the urethral folds.
- C: Development of glanular segments of the urethra.
- D: Conditions in the newborn.
Pathogenesis
Hypospadias is caused by the arrest of normal development of the urethra, at various stages of embryonic development (9 to 13 weeks of pregnancy). From the final shape of the anomaly, the time when the disturbance occurred can be predicted. In addition to the disturbances in the development of the urethra, the development of the raphe and the foreskin can also be compromised. This results in hypospadias with deviation from the raphe, the formation of a redundant dorsal prepuce foreskin and other ventral defects of the prepuce.
The penile shaft deviation can have several causes:
- Chordee: fibrous remnant of the corpus spongiosum distal to the meatus. It extends in a V shape on both sides, and acts by the lack of elasticity producing a ventral deformity.
- Dystrophic ventral penile shaft skin: frequently the ventral penile skin is dystrophic and inelastic. These skin components are connected by fibrous bridges to the deep penile fascia (Buck´s fascia) and the corpus spongiosum.
- Scrotal transposition: This term refers to the highly faceted scrotum (bifid scrotum), which shortens ventrally, due to a defect in the caudal migration of the scrotum during fetal life. It usually does not generate a real penile shaft deviation, only a visual effect, demonstrating a straight penile shaft during palpation, even with an erection. When it comes to a more pronounced defect, it can act as a barrier to erection.
- Faster growth of the posterior portions of the corpus cavernosum in comparison to the ventral parts: probably this type of penile curvation is the one presented in the special form of hypospadias, the sine hypospadias.
Diagnosis:
- Classification
- Clarification of associated anomalies:
- A patent processus vaginalis (9%)
- Undescended testes (5% in mild forms, 31% for proximal hypospadias)
- Anomalies of the upper urinary tract.
- Penoscrotal hypospadias in combination with undescended testes and scrotal transposition, or DDS require a complete genetic and hormonal evaluation.
- In severe hypospadias further urological examinations should be included, such as a Voiding cystourethrogram (MCU), to detect additional nephrourologic malformations.
- Physical examination and abdominal ultrasound, because of a possible association with kidney tumors in the more severe types of hypospadias.
Treatment options:




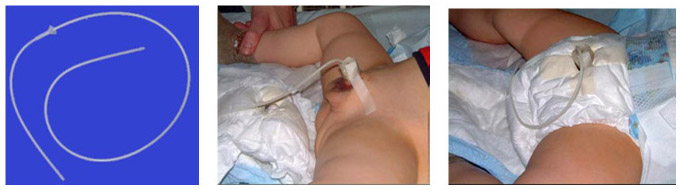
 Basically there are three different types of urinary diversion available:
Basically there are three different types of urinary diversion available: