Embryology of the Urinary Tract
Kathleen Kieran, MD; Christopher S. Cooper, MD
University of Iowa Department of Urology
→ Enlace a la versión en español
Kathleen Kieran, MD; Christopher S. Cooper, MD
University of Iowa Department of Urology
→ Enlace a la versión en español
An understanding of the embryology of the genitals and urinary system is integral to the comprehension of normal and pathologic function of these organs. While the genitals and urinary system have largely discrete functions, they share common embryologic origins.
Kidneys
The kidneys progress through three developmental stages: the pronephros, the mesonephros, and the metanephros. The pronephros develops in gestational week 3 as a condensation of intermediate mesoderm in the lower cervical and upper thoracic regions extending to the cloaca, and almost entirely regresses in gestational week 4 (Figure 1).
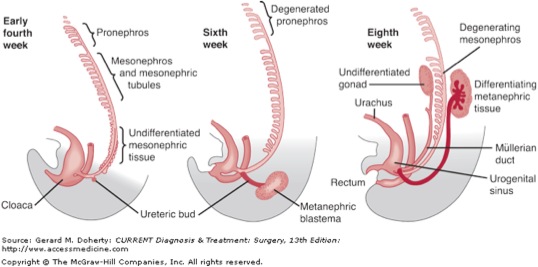
The pronephric duct, which arises from dorsal and caudal evaginations of the pronephros, is preserved and ultimately will give rise to the mesonephric duct. The mesonephros develops more caudally, from intermediate mesoderm; although the majority of these tubules degenerate, the mesonephric duct persists bilaterally. In both sexes, the ureters, renal pelvis, and bladder trigone are derived from the mesonephric duct; in the male, the mesonephric duct also gives rise to the vasa deferentia, epididymides, and seminal vesicles; the former is part of the duct itself, while the latter two structures arise as a result of ductal dilatation or outpouching (Figures 2, 3).
Once the mesonephric duct comes in contact with the cloaca at the caudal aspect of the embryo, it then grows cranially as the ureteric bud until it comes in contact with the metanephric mesenchyme, forming the metanephros.1
The ureteric bud and metanephric mesenchyme reciprocally induce growth, forming the kidney. The ureteric bud progressively enlarges and divides to form the renal pelvis, infundibula, collecting ducts, and 8-12 major and minor calyces.2 Branching of the ureteric bud is controlled by the interaction of glial-cell-derived neurotrophic factor (GDNF) and the RET-1 receptor, with subsequent activation of Wnt-11.3,4 Bone morphogenetic protein (BMP)-4 acts locally to decrease ureteric bud genesis and branching, and appears to be involved in preventing ureteral bud ectopia and promoting the development of periureteral smooth muscle5; gremlin 1 (grem 1), a BMP-4 antagonist, is normally present around the ureteric bud, locally decreasing BMP-4 activity and thus permitting ureteral branching.3 Numerous other genes and proteins have been implicated in normal and abnormal renal development.6 The collecting tubules invaginate metanephric mesoderm to form metanephric vesicles, which subsequently elongate to form metanephric tubules. As the metanephric tubules are invaginated by capillaries (glomeruli), nephrons are formed. This process continues until the 32nd gestational week. At birth, approximately 750,000 to 1 million nephrons are present in each kidney; postnatally, renal size may increase, owing to elongation of the proximal convoluted tubules.7
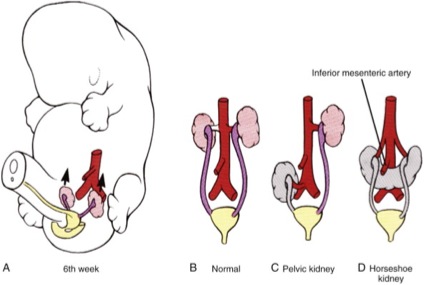
With differential longitudinal growth of the embryo, the kidney “ascends” from its initial location in the pelvis to its final location in the upper retroperitoneum. During ascent, transient blood vessels serially arise and degenerate; these arteries persist in ectopic kidneys as well as in some orthotopic renal units. Concurrently, the kidneys rotate around their vertical and horizontal axes so that their final orientation is one in which the upper poles are slightly more medial and anterior than the lower poles (Figure 4).8
Bladder
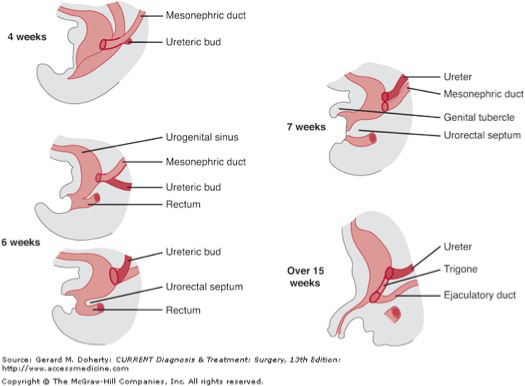
Until gestational week 7, the embryo has a cloaca, a single orifice at the caudal aspect. During gestational week 7, the urogenital membrane grows caudally, dividing the cloaca into ventral (urogenital sinus) and dorsal (rectum) components (Figure 5).
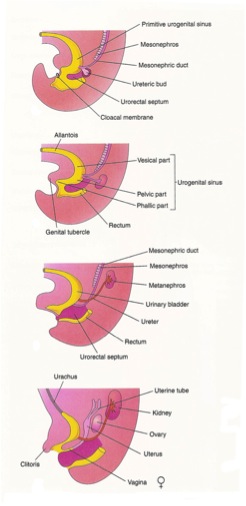
The urogenital sinus can be further subdivided into cranial (future bladder) and caudal (future prostate, urethra, and external genitalia) portions. The vesical epithelium is entirely derived from the endodermal layer of the urogenital sinus. As previously noted, the mesonephric duct gives rise to the ureter. With continued caudal growth of the embryo, the proximal (bladder) end of the mesonephric duct is progressively absorbed caudally, such that the common portion of the mesonephric duct is absorbed into the bladder trigone and urogenital sinus, and the discrete “branches” of the mesonephric duct destined to become the male genital ducts and ureters are now distinct entities attached to the urogenital sinus. Although the trigone is generally accepted to derive from the mesonephric ducts, the manner in which this is accomplished is not completely understood, and recent work suggests that the trigone may form primarily through vitamin A-mediated apoptosis of the common nephric duct rather than from true incorporation of the mesonephric ducts.9.10 An endodermal origin of the trigone has also been postulated.11
As the embryo continues to grow longitudinally, the orifices of the “branches” of the mesonephric ducts move progressively further apart, with the ureteral orifices moving craniolaterally and the male genital ducts moving caudally and medially. In cases where the origin of the ureteric bud is more distal on the mesonephric duct than normal, the incorporation of the mesonephric duct into the trigone does not yield two discrete ducts until much later in the process than is typical; as such, male genital ducts in this case usually have an orthotopic location, but the ureteral orifice is located more medially and caudally than usual. In severe cases, the origin of the ureteric bud may be so distal on the mesonephric duct that, even after incorporation of the mesonephric duct into the trigone, the ureteral orifice opens into the male genital ducts. Conversely, patients in whom the bifurcation of the mesonephric duct and ureteric bud is more proximal than usual typically have a ureteral orifice which is displaced laterally. Ureteric buds with significant ectopia are often associated with renal dysplasia.12,13
The nonepithelial layers of the detrusor (non-trigone) portion of the bladder arise from condensations of splanchnic mesenchyme. The lumen of the allantois, which connects the bladder and the anterior abdominal wall, closes over time, yielding the urachus. Over time, the urachus becomes more fibrotic and becomes the median umbilical ligament.1
Prostate
The prostate gland derives from the urogenital sinus, as endoderm invaginates into surrounding mesenchyme in gestational weeks 9-11 (Figure 6).14
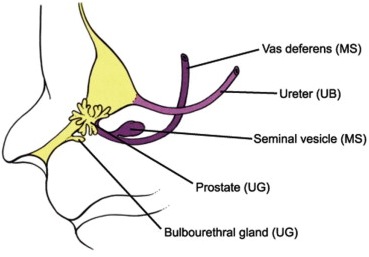
The gland enlarges and matures through the coinductive effects of mesenchyme and endoderm upon one another.15 Prostate development is an androgen-dependent process; it appears that the mesenchyme, rather than the endoderm, must be androgen-sensitive in order for normal prostatic development to occur.16 As with renal development, ongoing research continues to identify genes and proteins essential for prostatic development, including Shh, Sox-9, FGF, Wnt, Hox, and Fox.17
Urethra
The urethra is derived from the urogenital sinus, with endoderm giving rise to the epithelium and splanchnic mesenchyme giving rise to the surrounding soft tissue. In males, the most distal part of the urethra (the glanular portion) appears to arise from an ectodermal invagination which then joins with the endodermal epithelium of the proximal urethra to create a continuous channel (see Penis).18
Gonads
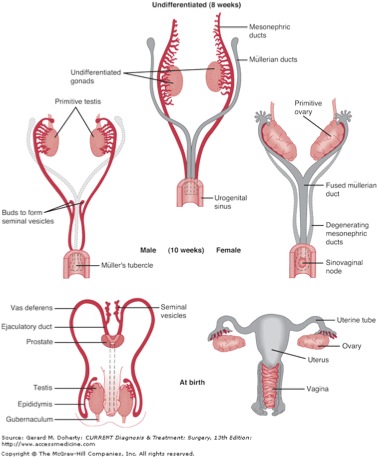
In the fifth gestational week, condensations of mesodermal epithelium and underlying mesenchyme form the gonadal ridges just lateral to the mesonephros. Primordial germ cells near the allantois on the ventral side of the embryo move dorsally as the embryo folds. These germ cells are taken up into clusters of epithelium called the sex cords, which invaginate the local mesenchymal tissue and migrate to the gonadal ridges. As these epithelial cells cluster together, distinct cortical and medullary rUntil the seventh week, the gonads of male and female embryos are identical. Further differentiation of the gonad into either a testis or ovary is determined at the most basic level by the chromosomal sex of the embryo (46,XY or 46,XX; Figure 7).
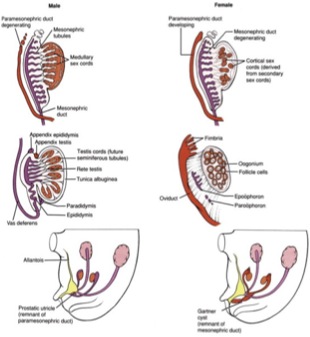
The testis-determining factor (TDF, also known as the Sry gene) on Yp (the short arm of the Y chromosome) is crucial for development of the undifferentiated gonad into a testis20; when TDF is present, the gonadal sex cords organize into future seminiferous tubules with a thickened capsule (tunica albuginea). The Sox-9 and DAX-1 genes are also involved in directing gonadal differentiation.21,22 The mesenchymal tissue between the seminferous tubules differentiates into Leydig cells, which synthesize testosterone in response to hCG stimulation between gestational weeks 8-12; the mesonephric (Wolffian) ducts and external genitalia are masculinized in response. The Sertoli cells of the testis, which originate from the surface epithelium under the apparent control of DAX-1, later become incorporated into the walls of the seminiferous tubules, secrete antimüllerian hormone (influenced by SF-1 and WT-1), which results in disintegration of the paramesonephric (Müllerian) ducts.23,24 Importantly, the seminiferous tubules do not develop a lumen until puberty.
Conversely, embryos with a 46,XX genotype or in whom TDF is not present will develop ovaries.25 Two X chromosomes are generally needed for normal ovarian development.26 Primary sex cords which extend into the medulla in a manner analogous to the development of the testis later degenerate.27 The secondary sex cords, which grow from the surface epithelium into the underlying mesenchyme, incorporate primordial germ cells, but later break into clusters called primordial follicles, each consisting of an oogonium: one germ cell surrounded by follicular cells from the sex cord; the number of follicles is augmented by prenatal mitosis (which does not continue postnatally). Although most oogonia degenerate before birth, those that do not will continue to grow and develop into primary oocytes.28
The development of the mesonephric ducts has already been discussed. The paramesonephric ducts, lateral to the mesonephric ducts, extend from the peritoneum to the future pelvis of the embryo. The paramesonephric ducts fuse in the pelvis, forming the uterus and upper two-thirds of the vagina in females (Figure 8).
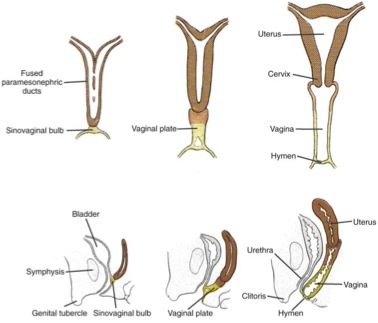
The unfused portions of the ducts become the fallopian tubes. Splanchnic mesenchyme gives rise to the uterine stroma and lining, while the epithelium of the vagina arises from urogenital sinus endoderm. A transverse septum divides the portions of the vagina derived from the urogenital sinus and Müllerian systems, and typically ruptures (leaving the hymen) shortly before birth.29-31
egions of the gonad become evident by the sixth gestational week.19
External Genitalia
Differentiation of the external genitalia into male and female variants begins in the seventh gestational week. A genital tubercle arises from condensations of mesenchyme near the embryonic cloaca in the fourth gestational week; on either side, labioscrotal (genital) swellings and urogenital (urethral) folds develop. The urorectal septum grows toward the cloaca, and by the end of the sixth gestational week has divided the cloaca into urogenital (anterior) and anal (posterior) portions.1
In the male, exposure to androgens results in growth of the genital tubercle to form a phallus, and the urogenital folds form the lateral borders of the urethral groove, and will ultimately fuse ventrally to cover the urethral plate (endoderm), which is contained within the urethral groove.32 The urethra is further covered by ectoderm. The development of the glanular urethra has been debated; one theory is that tubularization of the urethral plate from fusion of the labioscrotal folds continues distally in the same manner as it is accomplished proximally33, while another theory contends that ectodermal invagination of the tip of the glans penis with subsequent contact with the penile urethra gives rise to a fully canalized urethra.15 The former theory is currently believed to be correct. The paired corpora cavernosa and single corpus spongiosum arise from mesenchymal condensations.34,35 The scrotum derives from proximal fusion of the labioscrotal folds.15,36-38
In the female, there is minimal growth of the genital tubercle, which develops into the clitoris. Posteriorly, the urogenital folds fuse minimally to yield the posterior fourchette of the vaginal orifice; anteriorly, the unfused portions become the labia minora. The urogenital folds do not fuse and give rise to the labia majora. These morphologic changes may be mediated, at least in part, by the Shh gene.39
Outgrowth and branching of the urethral tissue into the surrounding mesenchyme gives rise to urethral glands and paraurethral (Skene’s) glands). The urogenital sinus has outpouchings which will become the Bartholin (vestibular) glands. The male analogs of these structures are the prostate (paraurethral glands) and bulbourethral glands (vestibular glands).
References.
1. Moore KL, Persaud TVN. The urogenital system. In The Developing Human: Clinically Oriented Embryology, 8th ed. Philadelphia: Saunders, 2003; chapter 12, pp. 243-283.
2. Yu, J., McMahon, A. P. and Valerius, M. T. (2004). Recent genetic studies of mouse kidney development. Curr. Opin. Genet. Dev. 14, 550-557.
3. Michos O, Panman L, Vintersten K, et al. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 2004;131:3401-10.
4. Majumdar A, Vainio S, Kispert A, et al. Wnt11 and REt/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 2003;130:3175-85.
5. Wang GJ, Brenner-Anantharam A, Vaughan ED, et al. Antagonism of BMP4 signaling disrupts smooth muscle investment of the ureter and ureteropelvic junction. J Urol 2009;181:401-7.
6. Stuart RO, Bush KT, Nigam SK. Changes in gene expression patterns in the ureteric bud and metanephric mesenchyme in models of kidney development. Kidney Int 2003;64:1997-2008.
7. Bertram JF, Douglas-Denton RN, et al. Human nephron number: implications for health and disease. Pediatr Nephrol 2011;26:1529-33.
8. Friedland GW, deVries P. Renal ectopia and fusion. Embryologic basis. Urology 1975;5:698-706.
9. Batourina E, Tsai S, Lambert S, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 2005;37:1082-9.
10. Viana R, Batourina E, Huang H, et al.The development of the bladder trigone, the center of the anti-reflux mechanism. Development 2007;134:3763-9.
11. Tanaka ST, Ishii K, Demarco RT, et al. Endodermal origin of bladder trigone inferred from mesenchymal-epithelial interaction. J Urol 2010;183:386-91.
12. Mackie GG, Stephens FD. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol 1975:114:274-80.
13. Schwarz RD, Stephens FD, Cussen LJ. The pathogenesis of renal dysplasia. II. The significance of lateral and medial ectopy of the ureteric orifice. Invest Urol 1981;19:97-100.
14. Thomson AA. Role of androgens and fibroblast growth factors in prostatic development. Reproduction 2001;121:187-95.
15. Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation 1999;64:115-22.
16. Lasnitzki I, Mizuno T. Prostatic induction: interaction of epithelium and mesenchyme from normal wild-type mice and androgen-insensitive mice with testicular feminization. J Endocrinol 1980;85:423-8.
17. Meeks JJ, Schaeffer EM. Genetic regulation of prostate development. J Androl 2011;32:210-7.
18. Krishnan A, de Souza A, Konijeti R, et al. The anatomy and embryology of posterior urethral valves. J Urol 2006;175:1214-20.
19. McKay DG, Hertig AT, Adams EC, et al. Histochemical observations on the germ cells of human embryos. Anat Rec 1953;117:201-19.
20. Berta P, Hawkins JR, Sinclair AH, et al. Genetic evidence equating SRY and the testis-determining factor. Nature 1990;348:448-50.
21. McElreavey K, Barbaux S, Ion A, Fellous M. The genetic basis of murine and human sex determination: a review. Heredity 1995;75:599-611.
22. Vilain E, McCabe ER. Mammalian sex determination: from gonads to brain. Mol Genet Metab 1998;65:74-84.
23. Rey R, Lukas-Croisier C, Lasala C, et al. AMH/MIS: what we know already about the gene, the protein and its regulation. Mol Cell Endocrinol 2003;211:21-31.
24. Rey R, Grinspon RP. Normal male sexual differentiation and aetiology of disorders of sex development. Best Pract Res Clin Endocrinol Metab 2011;25:221-38.
25. Hawkins JR, Taylor A, Berta P, et al. Mutational analysis of SRY: nonsense and missense mutations in XY sex reversal. Hum Genet 1992;88:471-4.
26. Simpson JL. Gonadal dysgenesis and abnormalities of the human sex chromosomes: current status of the phenotypic-karyotypic correlations. Birth Defects 1975;11:23-59.
27. Merchant-Larios H, Centeno B. Morphogenesis of the ovary from the sterile W/Wv mouse. Prog Clin Biol Res 1981;59B:383-92.
28. Oktem O, Oktay K. The ovary: anatomy and function throughout human life. Ann N Y Acad Sci 2008;1127:1-9.
29. Cunha CR. The dual origin of vaginal epithelium. Am J Anat 1975;143:387-92.
30. Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 2003;4:969-80.
31. Spencer TE, Dunlap KA, Filant J. Comparative developmental biology of the uterus: insights into mechanisms and developmental disruption. Mol Cell Endocrinol (in press).
32. Baskin LS, Erol A, Jegatheesan P, et al. Urethral seam formation and hypospadias. Cell Tissue Res 2001;305:379-87.
33. Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol 2008;318:143-52.
34. Baskin LS, Lee YT, Cunha GR. Neuroanatomical ontogeny of the human fetal penis. Br J Urol 1997;79:628-40.
35. Yiee JH, Baskin LS. Penile embryology and anatomy. ScientificWorldJournal 2010;10:1174-9.
36. Feldman KW, Smith DW. Fetal phallic growth and penile standards for newborn male infants. J Pediatr 1975;86:395-8.
37. Zalel Y, Pinhas-Hamiel O, Lipitz S, et al. The development of the fetal penis – an in utero sonographic evaluation. Ultrasound Obstet Gynecol 2001;17:129-31.
38. Kluth D, Fiegel HC, Geyer C, et al. Embryology of the distal urethra and external genitals. Semin Pediatr Surg 2011;20:176-87.
39. Miyagawa S, Matsumaru D, Murashima A, et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology 2011:152:2894-903.

